AmericanChemicalSociety.com
Reports: AC10 47787-AC10: Zeolite Encapsulated Poly(phosphaalkynes): A New Approach to an Elusive Target
Cameron Jones, Monash University
The success of the first year of this two year project has continued into its second year and excellent progress has been made towards its proposed goals, and related directions the project has taken. As research personnel funds remained at the end of the projects second year, it is the intention to capitalize on the early success of project by using these funds to partially support the research stipend of the graduate student most closely associated with it into a third year. This will be of great benefit to the project and will certainly lead to it generating a greater number of research outputs. In addition, the project continuity it will offer the graduate student involved will greatly benefit his research skill development and, thus, his future career prospects.
The original aim of the project was to initiate a completely new and fundamentally interesting area of chemistry, namely the linear polymerization of phosphaalkynes within the confines of zeolite hosts. The generated polyphosphaalkyne materials were expected to have unusual and potentially exploitable opto-electronic properties, based on their close relationship with well studied polyacetylenes. It should be noted that the encapsulation of conjugated polymers in zeolites (and all encapsulation technology) represents a completely new field for his group. In addition, Jones has no past experience of any note with the chemistry of "non-molecular" materials, or their exploitation. This project sees an expansion of the topical field of "modern main group chemistry" into the broader realm of materials chemistry, and is now having a significant impact on the PI's overall research portfolio. In addition, the research personnel associated with the project are learning valuable skills related to materials and solid state chemistries. These are being passed onto newer members of the research group working on related topics, thereby ensuring that that materials chemistry involving encapsulation technologies will continue to be a focus of the PI's group.
Further progress has been made towards optimizing dehydrochlorination preparative routes towards unhindered phosphaalkynes in the past 12 months. We can now routinely prepare multi-gram quantities of such compounds (e.g. PCR, R = H, Me, Et etc.) reproducibly. Their ready accessibility has allowed us to investigate the physical properties of these reactive monomers in detail. For example, we have initiated a collaboration with Prof. Don McNaughton of Monash University with whom we are investigating the far IR-spectral properties of PCMe at the Australian Synchrotron facility. It is expected that the results of this work will be published within 12 months. Our work from year 1 on the incorporation of phosphaalkynes within the pores of suitable zeolites, e.g. mordenite, has continued and we have found that this zeolite can act as "storage vessel" for PCMe, which largely remains associated with the zeolite pores under pressures of greater than ca. 2 atm., and does not significantly decompose within those pores at ambient temperature. Mordenite encapsulated PCMe does, however, appear to polymerize when this phosphaalkyne loaded material is irradiated with UV light. Combustion and TGA analyses suggest that the resulting orange material incorporates significant amounts of oligo- or polyphosphaalkyne within the zeolite pores (filling is estimated at > 30%). Further solid state 31P and 13C NMR spectroscopic studies on these materials have been carried out. The 31P NMR spectra exhibit broad peaks in the region 190-240 ppm, suggestive of the presence of P=C bonds in the included material. By altering the conditions of phosphaalkyne polymerization (e.g. temperature and UV exposure time), the extent of saturated P-centers in the polymerized materials, as indicated by high field (20-50 ppm) resonances in their 31P NMR solid state spectra can be minimized. These results provide good evidence for the formation of linear phosphaalkyne oligomers/polymers within the zeolite host, with the generation of small amounts of saturated cycloaddition products. In coming months, the characterization of the opto-electronic properties of these materials will continue. In order to qualitatively establish the effect that zeolite encapsulation has on phosphaalkyne polymerization, we have investigated the solid state spectroscopic properties of the completely insoluble brown/black material obtained when PCMe is treated with UV light. These show that the material consists of a complex mixture of phosphorus containing oligomers/polymers which are dominated by saturated P-centers.
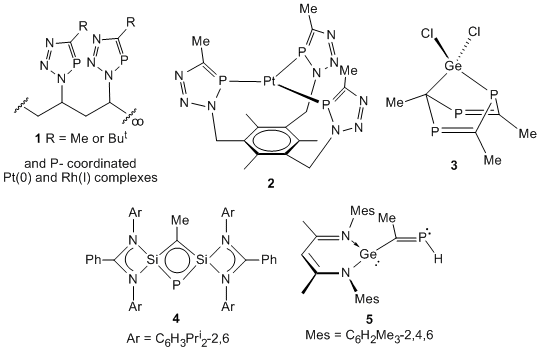
In related "spin-off" arms of this project we have continued to examine the catalyst free "click" [3+2] cycloaddition of phosphaalkynes with organoazide materials. This has allowed us access to a variety of polydentate and polymeric phosphatriazole materials, e.g. 1. These polymers are azide free, have MWs of 1800-2300 and narrow polydispersities, PDIs of ca. 1.8-2.0. They have been coordinated to potentially catalytically active transition metal fragments, as have the polydentate phosphatriazoles, e.g. in 2. Moreover, investigations into the use of low oxidation state group 14 compounds for the oligomerization of phosphaalkynes has led to phosphaalkyne trimerized products, e.g. 3, a remarkable phosphasilacyclobutadadiene species, 4, rare examples of hydrogermylated phosphaalkyne products, e.g. 5 etc. In the course of the later study a number of unusual phosphaalkyne free products have also been prepared, e.g. germa- and stannacyclopentadiene anionic heterocycles. Furthermore, the samarium(II) induced reductive dimerization of phosphaalkynes has been carried out in the past 12 months in our laboratory. The results of these studies have either been published recently, or will appear in a series of papers in the coming months.
Copyright © American Chemical Society