AmericanChemicalSociety.com
Reports: AC1 48564-AC1: Oxidative Palladium Coupling Chemistry
Craig A. Merlic, PhD, University of California (Los Angeles)
I. Overview
This project focused on developing new palladium(II) catalyzed cyclization strategies as improved methods for the synthesis of macrocycles. Enhanced synthetic efficiency was achieved by using an oxidative palladium(II) catalyzed coupling of boronate esters to access a broad range of cyclic structures containing diene and triene motifs from simple diyne precursors. By using a palladium(II) cycle, rather than a traditional palladium(0) mechanistic cycle, the process is environmentally friendly and operationally simple. The reactions tolerate an air atmosphere and occur at low temperatures since we avoid the high-energy oxidative addition step. A comparison of the palladium(0) and palladium(II) coupling mechanisms is shown in Scheme 1. The project successfully developed five types of cyclization strategies, and obtained limited results on two other types of cyclization methods. The project examined new methods, but is now ready for applications in the synthesis of biologically active or functional materials.
Scheme 1
II. Project
Achievements A. Palladium(II)
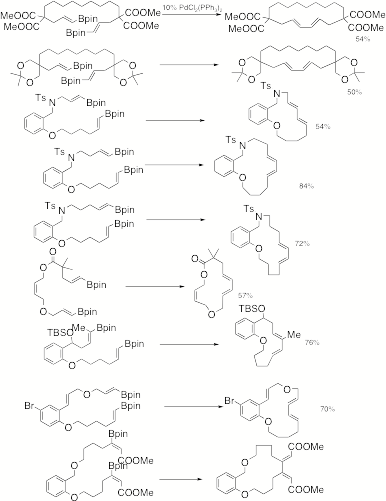
Catalyzed Macrocyclization to E,E-dienes. We
have completed a library of examples illustrating the ability to rapidly access
macrocyclic E,E-dienes (Scheme 2). The cyclizations tolerate a variety of
functional groups and are compatible with substituents on the vinyl boronate
substrates. A very important
example is the bromo substituted case which demonstrates that the palladium(II)
cyclization is compatible with substituents that are susceptible to oxidative
addition. This then allows a
palladium(II) catalyzed cyclization to be followed by a palladium(0) catalyzed
cross coupling. Scheme 2 We
were not able to develop reaction conditions to effectively use either air or
oxygen as the reaction reoxidant for palladium, though this is still under
investigation. The reactions
tolerated air, which has important process chemistry implications, but we
needed a more efficient oxidant for palladium such as chloroacetone or cupric
acetate. B. Palladium(II)
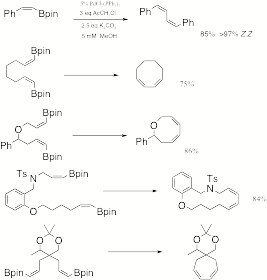
Catalyzed Macrocyclization to Z,Z-dienes. We
have also completed examples illustrating the ability to rapidly access cyclic Z,Z-dienes (Scheme 3). Like the E-vinylboronate esters, the
Z-vinylboronate esters are obtained in one step from alkynes. Thus in the fourteen-membered
macrocycle case we were able to make both the E,E and Z,Z products in two steps
from the same alkyne. These
results have important applications to natural products synthesis. Scheme 3 C. Palladium(II)
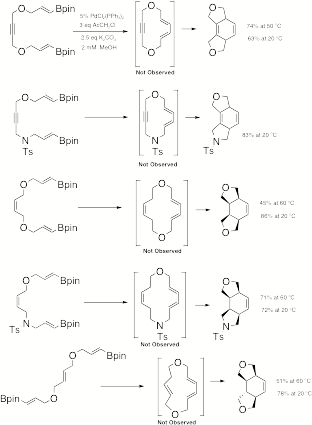
Catalyzed Macrocyclization to Transannular Diels-Alder Substrates. Our
chemistry provides a powerful method for the preparation of Transannular
Diels-Alder (TADA) substrates and we have completed five systems (Scheme 4) and are almost finished with two more. All of the cases shown underwent
spontaneous cyclization due to ring strain activation, so the two cases we are
working on will have larger macrocyclic rings, so the TADA substrate will be
observed. The palladium(II) route
is quite efficient and also very mild, allowing cyclization at room temperature
which usually resulted in higher yields. Scheme 4 D. Palladium(II)
Catalyzed Macrocyclization to Alkenes. Suzuki
cross coupling reactions employing alkyl boranes, rather than boronic acids or
boronate esters, as nucleophilic components are now well established, so we
sought to translate that technology into a cyclization of a-vinyl w-alkyl
boranes providing a cyclic alkene product. That proved overly optimistic as we were only able to obtain
trace amounts of alkyl-alkenyl coupling under a variety of reaction conditions
(Scheme 5). A key requirement for this type of cyclization to be
successful is for alkenyl transmetallation to be faster than alkyl
transmetallation in order to avoid beta-hydride elimination. E. Palladium(II)
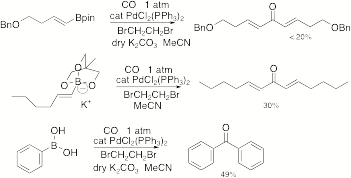
Catalyzed Carbonylative Macrocyclization to E,E-Dienones. Many
biologically active macrocycles contain ketone moieties and often they are
conjugated to alkenes, thus we sought a carbonylative oxidative palladium
catalyzed coupling of the vinyl boronates. Unfortunately, an optimal process has proven to be
elusive. This is surprising given
other known carbonylative palladium catalyzed cross coupling reactions. We only have limited results with some
intermolecular test cases (Scheme 6),
so this important topic is still under investigation. Scheme 6 F. Palladium(II)
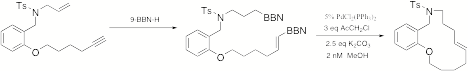
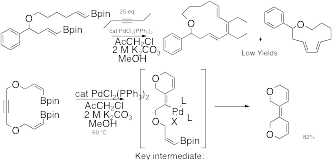
Catalyzed Cyclization with Alkyne Insertion. Macrocyclization
with concurrent alkyne insertion is mechanistically straightforward, but the
challenge is actually a matter of reaction rates. Every macrocyclization must be performed at high dilution to
avoid polymer formation, but insertions of small molecules require high
concentrations of the inserting component. We have not yet found conditions that succeed for
intermolecular cases, but the intramolecular system works exceedingly well (Scheme
7). Scheme 7 G. Palladium(II)
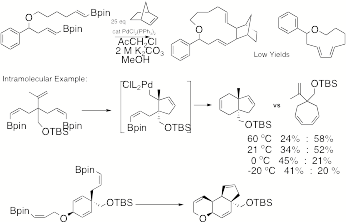
Catalyzed Cyclization with Alkene Insertion. Like
alkyne insertion reactions, intermolecular alkene insertion reactions are
mechanistically simple, but practically challenged by the issue of reaction
rates. And like the alkyne
insertion reactions, the intramolecular cases proved successful (Scheme 8).
However, even here we saw a competition between alkene insertion and
direct cyclization. Exploring that
aspect further we discovered two fascinating details. First, there was a strong temperature dependence on the
ratio of reaction pathways.
Second, we were able to achieve cyclization couplings at a remarkable
-20 oC. That has
extremely important implications for diastereoselective and enantioselective
palladium catalyzed cyclizations that we are now pursuing using the last system
illustrated. Scheme 8 III.
Educational Components The
grant supported the research activities of four undergraduate and five graduate
students. Two undergraduate
students graduated and one of them went on to graduate school in chemistry. Two
undergraduate students are currently working on the project and both will go on
to graduate school in chemistry.
Five graduate students worked on this project as a significant component
of their PhD theses and all will graduate with their degrees by next year. IV. Summary The
project has been hugely successful and has opened up several avenues for
further exploration. We are in the
process of putting together three communications and one full paper based on
the results obtained through the support from this grant. There was a delay in having the papers
already complete due to a personnel issue, but we should have all papers
submitted over the next few months.
The results obtained through the support of this grant are being used to
support a major grant application.
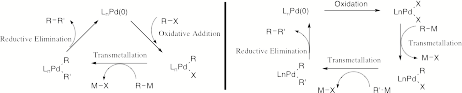
Copyright © American Chemical Society