AmericanChemicalSociety.com
Reports: UR1 49436-UR1: Cellulose Dissolution and Hydrolysis in Acidic Ionic Liquids
Ananda S. Amarasekara, Ph. D, Prairie View A&M University
The goal of the project is to develop an environmentally sound and industrially feasible cellulose hydrolysis method for the cellulosic-ethanol process. This goal would be achieved by the development of a recyclable acidic ionic liquid solvent system that can easily dissolve cellulose and act as the hydrolysis catalyst. The milestones targeted for the period were (a) Synthesis and characterization of six acidic ionic liquids and (b) Studying the dissolution and hydrolysis of cellulose in these acidic ionic liquids.
The initial step of the project involved the synthesis of acidic ionic liquids (1-3) (Figure 1) with a sulfonic acid arm by condensation of the corresponding nitrogen bases with 1,3-propane or 1,4-butane sultones and then acidification of the salts with HCl. These ionic liquids were characterized by NMR spectroscopy.
Figure1. Acidic ionic liquids (1-3)
Dissolution of cellulose in these
ionic liquids were tested at room temperature by mixing different cellulose
samples and ionic liquids in glass vials and slow dissolving samples were
allowed to stand at room temperature after initial mixing. Hydrolysis was
carried out with or without preheating, by adding a controlled amount of water
such that no precipitation of cellulose occurs, and warming the vials in a
thermostated water bath. Next, the samples were diluted with water, neutralized,
and then centrifuged to give clear supernatant solutions. These solutions were
analyzed for total reducing sugars (TRS) using 3,5-dinitrosalicylic acid (DNS) method, and glucose by
the Glucose Oxidase/Peroxidase
assay using a Sigma GAGO-20 kit.
Dissolution of five different types cellulose samples in acidic ionic liquids was studied.
The types of cellulose studied are; a-cellulose
(DP ~ 100), microcrystalline (MC)-cellulose (DP ~ 240), Sigmacell cellulose (DP
~ 450), Whatman filter paper, and cotton wool. All five types of cellulose
samples are found to dissolve in imidazolium type Brönsted acid ionic liquids (1a, b) within 2-5 min. at room
temperature and atmospheric pressure. These
cellulose samples were found to dissolve in triethanolammonium (2a,b) and
pyridinium ionic liquids (3a, b)
when allowed to stand at room temperature for 24 hrs. Total amounts of reducing sugars
and glucose formed in selected experiments are shown in the Table 1.
________________________________________________________________________
Temperature ( °C) /time (min) Yield (%)
before after
Entry
IL/Cellulose
adding H2O adding
H2O TRS
glucose
________________________________________________________________________
1 1a/a-cellulose 70/60 70/30 59 15
2 1a/MC-cellulose 70/60 70/30 12 4
3 1a/Sigmacell 70/60 70/30 62 14
4 1a/Sigmacell - 70/30 39 12
5 1a/Sigmacell 70/40 70/30 56 12
6 1a/Sigmacell 70/60 70/60 42 7
7 1a/Sigmacell 70/60 70/240 29 4
8 1a/Sigmacell - 50/960 32 3
8 1a/Sigmacell - 90/30 34 3
10 1a/Sigmacell 90/30 90/30 26 2
11 1a/Sigmacell - 90/240 15 1
12 1b/a-cellulose 70/60 70/30 32 -
13 1b/MC-cellulose 70/60 70/30 7 -
14 1b/Sigmacell 70/60 70/30 12 -
12 3b/a-cellulose 70/60 70/30 32 -
13 3b/MC-cellulose 70/60 70/30 7 -
14 3b/Sigmacell 70/60 70/30 12 -
________________________________________________________________________
Table 1.
Average % yields of TRS and glucose produced in duplicate experiments. 10% w/w
Cellulose in the acidic ionic liquid solutions, 2.0 equivalents of H2O
per glucose unit of cellulose were added in all hydrolysis experiments Hydrolysis experiments using
triethanolamine based acidic ionic liquids (2a, b) failed to give any reducing sugars. Immidazolium acidic
ionic liquids gave better yields than pyridinium ionic liquids and
1-(1-propylsulfonic)-3-methylimidazolium chloride (1a) medium produced
the highest yields of TRS and glucose with most of the cellulose samples
studied. Both a-cellulose and Sigmacell
cellulose produced moderate TRS yields, whereas microcrystalline (MC)
cellulose, Whatman filter paper, and cotton wool produced poor yields in all of
the hydrolysis experiments. The experiment with 1a/Sigmacell cellulose solution showed
that highest TRS (62%) and glucose (14%) yields, and was attained with 1hr. preheating at 70 °C and 30 min. heating at 70 °C, after adding water, as shown in the entry 3. Heating the
sample at lower temperatures for a longer time (entry 8) or heating for a
longer time at 70 °C (entry 6, 7) failed to
give better yields. Furthermore, longer
heating times (entry 7) and higher temperatures (entry 10, 11) produced
excessive charring of the sample, giving black residues.
In conclusion, key milestones were
achieved for the first year of the project. The proposed forward path for the
second year involves the following steps: (a) Optimization of the TRS and
glucose yields (b) Testing the acidic ionic liquid dissolution and hydrolysis
method on untreated raw biomass forms such as switchgrass and poplar (c) Recovery
and reuse of the acidic ionic liquids.
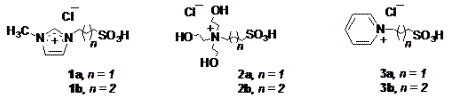
Copyright © American Chemical Society

