AmericanChemicalSociety.com
Reports: DNI2 49028-DNI2: A New Hypothesis for Interpreting the Sedimentary Cerium Anomaly Paleo-Redox Proxy in Oil Shale Depositional Environments: The Influence of Particulate Carrier Fluxes (Mn, Fe, Algal Tissue)
Dr. Johan Schijf, University of Maryland Center for Environmental Science
Yttrium and the rare earth elements (YREEs) form a unique series of trace metals. Extremely similar properties make them hard to separate by chemical and physical processes and, in environmental samples, they are always found together as a group. All YREEs are trivalent in natural waters and their ionic radii decrease gradually from the lightest element (La) to the heaviest (Lu), whereas Y generally resembles the heavier REEs. These attributes combined cause subtle fractionations among the YREEs that are widely employed as a powerful diagnostic tool in studies of various geochemical processes, for example planetary evolution, ore formation, ground water provenance, global ocean circulation etc. An exception to this coherent behavior is cerium, being the only REE that oxidizes, within the range of temperatures and pressures found near the Earth surface, around the redox potential of the important Fe(II)/Fe(III) and Mn(II)/Mn(IV) couples. This is significant, because Ce(IV) is much more surface-reactive than Ce(III). Cerium partitioning between aqueous solutions and suspended particulate matter is therefore different from that of its REE neighbors, giving rise to distinctive Ce anomalies' in the normalized elemental abundance patterns of sedimentary phases that contain YREEs adsorbed from ambient seawater.
It is commonly assumed that enhanced Ce sorption on settling marine particles, compared to the YREE series as a whole, is due to its oxidation by dissolved oxygen and that the resulting sediments accordingly provide a lasting record of bottom water oxygenation at their time of deposition. Episodic shifts in sedimentary Ce anomalies have been interpreted as periods of anoxia, whereby positive Ce anomalies or their absence are purported to reflect oxidizing and reducing bottom water conditions, respectively. Prolonged anoxia promotes the preservation and burial of algal debris and other organic matter, which may ultimately become the source material for massive oil shale formation, and is therefore of considerable interest. The sedimentary Ce anomaly paleo-redox proxy' falters however if Ce(III) can be oxidized at the particle surface in the absence of dissolved oxygen, as is known to happen for instance on hydrous manganese oxide (HMO).
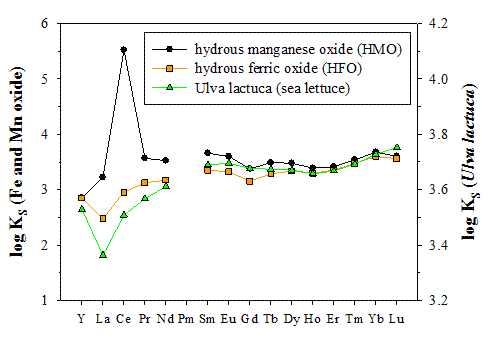
In my laboratory, M.Sc. student Kathleen Marshall investigates YREE sorption on three major components of marine particulate matter: hydrous ferric oxides (HFO), HMO, and algal debris. Her first approach has been to characterize sorption on the pure end members (see Figure). YREE sorption on HFO is mediated by hydroxyl groups that bind the YREEs in a monodentate or bidentate fashion, either as free cations or as ternary chloride and hydroxide complexes. Cerium tracks the other YREEs and the lack of a Ce anomaly indicates that no Ce oxidation occurs. These observations are accurately described with a designated non-electrostatic surface complexation model (Marine Chemistry, in press). YREE sorption on HMO is broadly similar to HFO, yet set apart by a pronounced positive Ce anomaly. Since these experiments are performed under anoxic conditions, this is evidence for Ce oxidation at the HMO surface in the absence of dissolved oxygen. YREE sorption on algal debris, which like HFO produces no Ce anomaly, has been investigated by M.Sc. student Alison Straka (unpublished data). New surface complexation models are being developed to describe the HMO and algal data. During the remainder of this project, Kathleen will study YREE sorption on mixtures of HFO and HMO and of algal debris and HMO, in different proportions. If the size of the Ce anomaly is directly related to the fraction of HMO in the mixture, this will be clear confirmation that Ce is an unreliable paleo-redox proxy and that interpretations of sedimentary Ce anomalies in terms of past changes in ocean bottom water oxygenation must be undertaken with appropriate caution.
Figure. Solid-solution
distribution coefficients (KS) for YREE sorption on HFO, HMO, and
algal debris, in 0.5 M NaCl at T = 25°C. Patterns were vertically displaced to
emphasize both the congruence for the heavier elements (Y, and SmLu) and the divergence for the lighter ones. The
anomalously strong sorption of Ce on HMO is due to its oxidation at the
particle surface. This phenomenon is not observed for HFO and the macroalga.
Copyright © American Chemical Society

