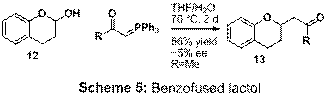AmericanChemicalSociety.com
Reports: GB1 48256-GB1: Enantioselective Nucleophilic Catalysis for the Synthesis of beta-Lactams and other Nitrogen Containing Heterocycles from Isocyanates
James Arthur MacKay, PhD, Elizabethtown College
During the course of the first year of funding an efficient synthesis of substrate 5 was achieved (Scheme 1). Efforts to cyclize 5 and related anologues continue, though attempts to promote the desired cyclization via nucleophilic catalysis have not resulted in the formation of bicyclic β-lactams. Evidence exists for the initial attack of the nucleophile into the isocyanate, however the key conjugate addition step has not taken place.
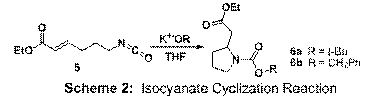
Stoichiometric alkoxides
can promote cyclization. Treating 5 with potassium tert-butoxide led to the
formation of 6a in 35% yield and
using potassium benzyloxide gave 6b in 19% yield (Scheme 2). Unfortunately,
mass balances have been problematic and possible polymerization pathways are likely
culprits. Interestingly, we have
discovered that acyclic carbamates form in high yield
using lithium alkoxides (rather than potassium). Stronger electron withdrawing groups
than the ethyl ester group may be needed to promote the C-N bond forming step. Isocyanates
tethered to more electron poor alkenes including nitroalkenes,
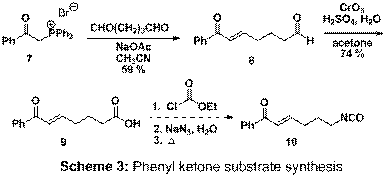
unsaturated nitriles, and ketones will be prepared. Our most current route uses phosphonium salt 7
with sodium acetate in acetonitrile to form a phosphonium ylide that reacts
with aqueous gluteraldehyde (Scheme 3) affording 8. Subsequent
oxidation of 8 affords carboxylic
acid 9 in 74% yield. Following a Curtius rearrangement we will
test the key cyclization reaction.
Successful cyclization will afford the opportunity to examine the
electronic effects of the initial Michael acceptor by varying substituents on
the aromatic ring in 7. Another project, which was spun off of
an undesired side reaction in efforts to make 9, involves the Wittig reaction of phosphonium
ylides and lactols (Table 1).
We have
explored the use of chiral amine catalysts in the synthesis of 2-substituted pyrans which are abundant in natural products and
pharmaceuticals. A variety of known chiral
amine catalysts were screened under several reaction conditions that afford 11. In each case, there was a small but
noticeable increase in rate compared to uncatalyzed
reactions. Unfortunately, enantioselectivities were low in all examples. Entry Cat. Solvent Temp (°C) % yield %ee Entry Cat. Solvent Temp (°C) % yield %ee 1 a THF 65 42 5 6 c benzene 70 53 <5 2 a H2O 65 15 <5 7 ca benzene 70 90 <5 3 a toluene 110 53 <5 8 d THF/H2O rt 30 <5 4 a benzene 70 80 <5 9 e THF/H2O rt 30 <5 5 b benzene 70 53 <5 10 e THF/H2O 70 70 7
Additional efforts to affect an
enantioselective Wittig reaction have not yielded enantioenriched
products. Neither the methyl ketone Wittig
reagent nor the aldehyde led to respectable yield using 2. A benzofused
substrate (12) was attempted with
the expectation that the intermediate phenol would be less nucleophilic than an
alcohol. Using substrate 12 with the methyl ketone Wittig
reagent (R = Me), the product 13 was obtained in good yield however with low enantiomeric
excess (Scheme 5). A
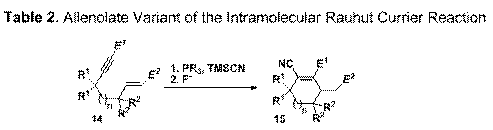
final project involves the intramolecular Rauhut-Currier
Reaction which was prompted by our interest in nucleophilic catalysis. The Rauhut-Currier reaction is often quite slow resulting in
the need for highly reactive alkyl phosphines and high catalyst loadings. To address this, our variant uses a reactive
alkyne. In this process, an external pronucleophile is required to regenerate the phosphine
catalyst. In a preliminary experiment, 14a was treated with catalytic nBu3P and 1.5 equivalents of
TMSCN resulting in a rapid reaction.
Following treatment with aqueous CsF,
the product 15a was isolated in 61%
yield. This encouraging result has led
to an effective method to construct a variety of highly functionalized carbocycles. We have
prepared and cyclized a range of enynes
(14a-14g) using alkyl phosphines
with TMSCN. Both 5- and 6-membered rings
can be formed. entry substrate n R1 R2 E1 E2 catalyst (%) yield, % 1 14a 0 H H CO2Me CO2Me PBu3 (10) 61 2 14b 0 H H CO2Me CN PBu3 (15) 41 3 14b 0 H H CO2Me CN PMe3 (15) 47 4 14c 0 CH3 H CO2Me CO2Me PBu3 (15) 39 5 14d 0 CH3 H CO2Me CN PBu3 (15) 37 6 14e 0 CH3 H CO2Me CN (Z) PBu3 (15) 31 7 14f 0 CH3 H SO2Ph CN PBu3 (15) 50 8 14g 0 H CH3 CO2Et CO2Et PBu3 (25) 37 9 14h 1 H H CO2Me CO2Me PBu3 (15) 50 10 14i 1 H H CO2t-Bu CO2t-Bu PBu3 (25) 40 This work has had significant impact in
addition to the science discussed above.
Since the funding of this proposal, 10 undergraduate students have been
trained in my research laboratory to date.
The learned skills and techniques are invaluable toward their future
endeavors as scientists. Three of the
students have graduated. One went to
graduate school, one to pharmacy school and one is teaching high school
chemistry. In addition, this work has
helped establish a foundation for several projects which will lead to an
extensive long term research program in organic synthesis and catalysis. The project has also initiated contact with
two collaborators that will broaden the impacts of the work.

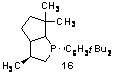 Additionally, we
have attempted an enantioselective reaction using a chiral phosphine. The Vedejs PBO catalyst (16) affords cyclization yields that are comparable to those in
using achiral catalysts. However, enantioselectivity was low ranging from racemic to 28% ee in three examples.
We postulated that low enantioselectivy could
be due to an unexpected mechanistic pathway.
To this end, a control experiment using 14f and catalytic BnMe3NCN
in the absence of a phosphine was attempted.
Under the normal reaction time, no reaction was observed thus
eliminating the possibility that cyanide is the catalytic species. We next postulated that racemization
could be occurring under the reaction conditions. In fact, proton transfer of the alkene
β-hydrogen is precedented and would lead to the
formation of a stable phosphonium ylide
intermediate in which the chirality center is
destroyed. We have refuted this
hypothesis by synthesizing a deutrated version of 14g.
Preliminary results suggest that this proton transfer is not occurring.
Additionally, we
have attempted an enantioselective reaction using a chiral phosphine. The Vedejs PBO catalyst (16) affords cyclization yields that are comparable to those in
using achiral catalysts. However, enantioselectivity was low ranging from racemic to 28% ee in three examples.
We postulated that low enantioselectivy could
be due to an unexpected mechanistic pathway.
To this end, a control experiment using 14f and catalytic BnMe3NCN
in the absence of a phosphine was attempted.
Under the normal reaction time, no reaction was observed thus
eliminating the possibility that cyanide is the catalytic species. We next postulated that racemization
could be occurring under the reaction conditions. In fact, proton transfer of the alkene
β-hydrogen is precedented and would lead to the
formation of a stable phosphonium ylide
intermediate in which the chirality center is
destroyed. We have refuted this
hypothesis by synthesizing a deutrated version of 14g.
Preliminary results suggest that this proton transfer is not occurring.
Copyright © American Chemical Society