AmericanChemicalSociety.com
Reports: DNI1 49226-DNI1: Metal-Catalyzed Addition of Aminoborane Reagents to Olefins
Seth B. Herzon, PhD, Yale University
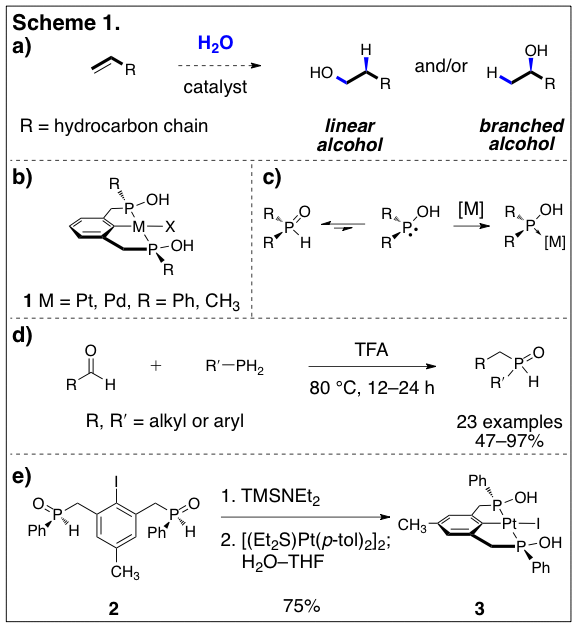
The direct addition of the elements of water to terminal olefins to form primary and secondary alcohols under mild conditions is a long-standing unsolved problem in catalysis (Scheme 1a). This reaction, often referred to as olefin hydration, converts simple hydrocarbon starting materials to oxygen-functionalized products with complete atom economy. Successful realization of such a process would broadly impact both commodity and fine chemicals synthesis. Nonetheless, mild catalytic methods for the hydration of olefins do not exist, although several efficient indirect methods have been developed.
Our laboratory has been focusing on creating a family of new late transition metal complexes and evaluating their suitability as catalysts for the hydration of olefins. The complexes we have targeted contain novel bis(phosphinous acid) PCP pincer ligands bound to Group 10 transition metals (Scheme 1b). Our rationale for targeting such complexes stems from several recent reports that established the utility of late metal pincer complexes in promoting the stoichiometric and catalytic additions of heteroatom-based nucleophiles to alkenes. However, these studies utilized ligands with bulky aryl- or alkyl-substituents on phosphorous. We reasoned that these substituents generate a sterically encumbered, hydrophobic pocket about the metal center that hinders the addition of small, polar nucleophiles to coordinated alkenes. Consequently, we targeted a ligand framework that would contain polar hydrophilic substituents on phosphorous, and identified pincer complexes of bis(phosphinous acids) as uniquely suited for this purpose. In solution, phosphinous acids exists as their air-stable P(V) secondary phosphine oxide tautomer, but coordinate to a metal center by conversion to the P(III) phosphinous acid form, as shown as shown in Scheme 1c. The complexes so formed (e.g., 1) contain small, polar hydroxyl substituents that are expected to result in effective hydration of the metal catalyst, increasing the local concentration of water and diminishing the enthalpic costs associated with approach to the complexed substrate. The PCP pincer ligand also creates a well-defined coordination sphere about the metal and inhibits the formation of multiple open coordination sites, which can lead to problematic side reactions, such as b-hydride elimination. Finally, the modular nature of the pincer ligand will allow systematic modification of the steric and electronic properties of our complexes.
In order to prepare these complexes we required synthetic access to complex secondary phosphine oxides. When we began our studies such ligands were beyond the scope of existing methods. We set out to develop a procedure for the synthesis of complex secondary phosphine oxides, and uncovered a simple, one-step protocol that involves heating of a primary phosphine with an aldehyde in trifluoroacetic acid to form the secondary phosphine oxide product in high overall yield in a single step (Scheme 1d). Germane to our proposed research, bis(secondary phosphine oxides) can now be synthesized in a single step in good yield from the dialdehyde precursor. More broadly, because secondary phosphine oxides are established precursors to tertiary phosphines, we believe this method may find use in the synthesis of other phosphorous-containing reagents.
With access to the requisite ligands, we have begun to synthesize our targeted metal complexes. In preliminary studies, we have successfully created the complex 3 from the bis(phosphine oxide) ligand 2 (Scheme 1e) We are currently expanding the scope of our complexes to incorporate ligands bearing P-methyl and P-cyclohexyl substituents. Once the requisite complexes are in hand, we will be in position to evaluate their efficiency in promoting the stoichiometric addition of water to alkenes, and ultimately, we will use this information to develop a catalytic process.
Copyright © American Chemical Society