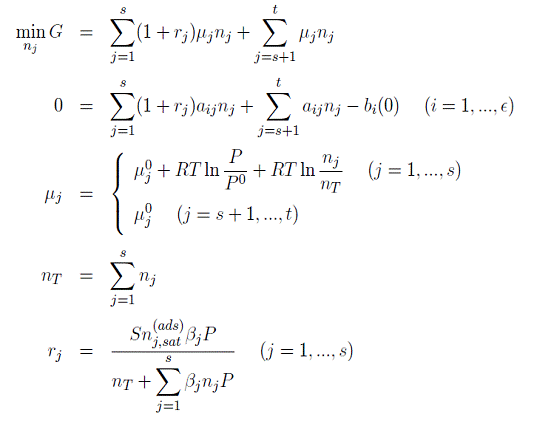AmericanChemicalSociety.com
Reports: UR5 50095-UR5: Adsorption of CO2 by MgO Aerogels for Enhanced Steam Reforming
Mingheng Li, PhD, California State Polytechnic University
Introduction
Adsorption Enhanced Reforming (AER) is a novel steam reforming technique that enables the production of fuel-cell grade hydrogen in a single step. When CO2 is removed from the reforming process, the water gas shift (WGS) reaction (CO + H2O = CO2 + H2) shifts to the right which results in a relatively lower reforming temperature and also an enhancement of the yield and purity of H2. The major CO2 adsorbents reported in AER are hydrotalcites (Mg6Al2(CO3)(OH)164H2O) and CaO-based materials. This research project is to investigate the potential application of MgO aerogels as a new adsorbent at low temperatures. The research aspects include materials synthesis and characterization as well as thermodynamic modeling.
Synthesis and Characterization of MgO Aerogels
In order to study the effects of surface defects, dopant modification, and composition on CO2 absorption of sol-gel derived MgO and MgO composites, the following materials were synthesized and characterized.
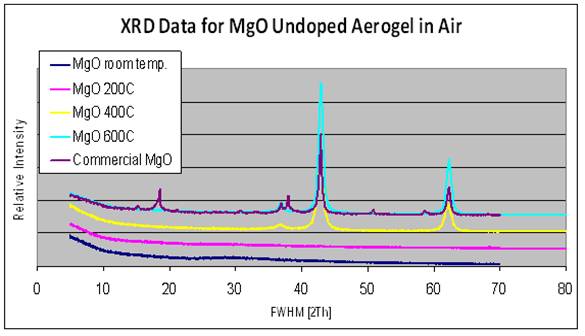
Amorphous MgO aerogels (with surface areas ~ 500 m2/g) and xerogels (with surface areas ~ 100 m2/g) were synthesized through the sol-gel process using a modified Pechini process. The MgO aerogels and xerogels are amorphous and crystallize to the periclase phase at ~ 450°C in air, without passing through the brucite phase (Figure 1).
Figure 1: XRD shows that sol-gel derived MgO are amorphous and
crystallize to the periclase phase at ~ 400°C. The commercial MgO contains both
brucite and periclase forms.
This is important to note since the crystalline MgO synthesized
from sol-gel derived aerogels and xerogels are purer forms of MgO, whereas
commercially available MgO contain traces of the brucite (Mg(OH)2)
phase. The periclase phase has XRD peaks at 2¦È = 38o, 43o,
and 62° whereas the brucite phase has primary peaks at 2¦È = 19o and
39°.
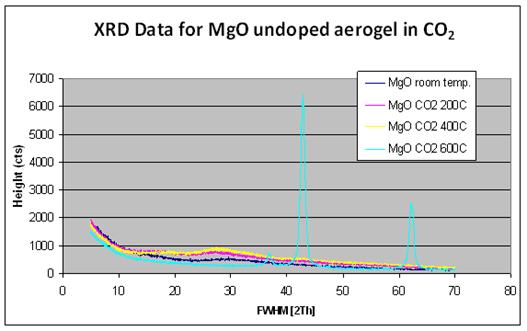
When sol-gel derived MgO were heat treated in CO2
instead of air, the transition to the periclase phase was delayed until 600°C
(Figure 2). Brucite formation was also not observed for this treatment process.
Figure 2: XRD of sol-gel derived MgO
heat-treated under CO2.
MgO doped with 3 mol% of Ni and 2 mol% of Co were also
synthesized. Neither dopants changed the crystallinity of the MgO matrix and
were too low in concentration to be detected with XRD. For CO2
adsorption between ambient to 800°C a thermogravimetric analyzer (TGA, TA
Instruments, SDT 2960) will be used. Preliminary studies with TGA under air
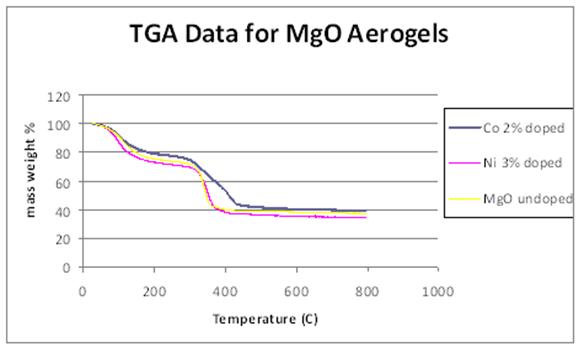
were conducted to determine material evolution with heat treatment. Figure 3
shows that all samples lost approximately 60% of their initial mass due to loss
of surface adsorbed water, organic solvents (80 - 180°C, physisorbed water and
organic solvents (300 - 400°C). Doping with Ni did not change the mass loss
profile of the MgO. Doping with Co appeared to slow the desorption process. A
similar study under wet-CO2 and dry-CO2 will be conducted
to determine the adsorption behavior of these materials.
Figure 3: Mass loss profiles of MgO, Ni-doped
MgO, and Co-doped MgO.
Current work includes synthesizing Rh-doped MgO as well as a
sol-gel derived MgO-Al2O3 composite that is similar to
hydrotalcite but with higher surface areas and exhibit metastable phases not
available through the traditional synthesis process.
Thermodynamic analysis of CO2 adsorption on steam
reforming A thermodynamic model has been developed to study the effect of
CO2 adsorption on steam reforming. The model is based on the
minimization of Gibbs free energy of the reactive system with the assumption of
Langmuir isotherm:
The above model was applied to the steam reforming of ethanol
at T = 500°C, P = 5 bar and molar ratio H2O/EtOH = 3. The model was
solved based on Langrage multiplier method for and some of the results are
summarized below.
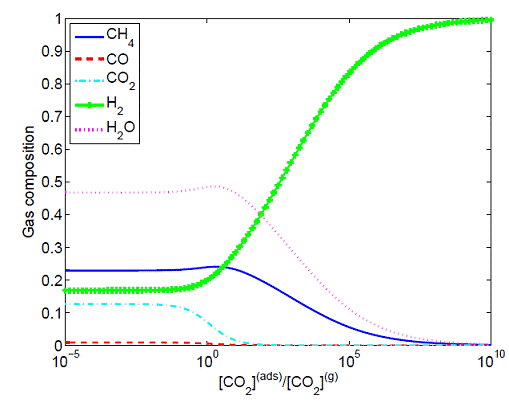
The gas composition in the product mixture under different CO2(ads)/CO2
(a parameter approximately proportional to the capacity of the adsorbent) is
shown in Figure 4. As CO2(ads)/CO2 increases,
the fraction of CO2(g) rapidly decreases to a minimal level (at CO2(ads)/CO2=101~2),
beyond which a significant reduction in the CO or CO2 concentration
is not obvious. The low partial pressure of CO2 in the gas phase
makes it difficult for a further adsorption. When CO2 is gradually
adsorbed on the surface, the forward reaction in the reversible water gas shift
reaction CO + H2O = CO2 + H2 is favored. As a
result, part of the CH4 is converted to CO through the methane
reforming reaction CH4 + H2O = CO + 3H2. In
this sense, the entire system behaves like a buffer solution. Due to the
coupling of multiple equilibrium phenomena, a complete removal of CO2
is very difficult. However, the fact that the primary carrier of carbon in the
gaseous phase is CH4 (instead of CO or CO2) at CO2(ads)/CO2
> 101 implies that a complete removal of CO2 is not
necessary.
Figure 4: Gas compositions of ethanol reforming at equilibrium
as a function of CO2(ads)/CO2 (H2O/EtOH
= 1/3, P = 5 bar and T = 500°C).
A parametric analysis based on Eq. (1) was made in a wide range
of operating conditions such as temperature, pressure, steam-to-carbon ratio and
with/without the presence of oxygen. It was shown in all cases that CO2
adsorption not only enhances H2 yield and purity, but also
suppresses the formation of coke, an undesired material that could deactivate
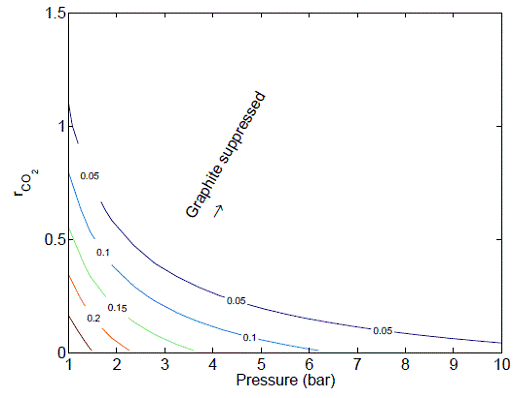
the reforming catalyst. For example, Figure 5 shows clearly that the coke
formation is suppressed by increased CO2 adsorption at a fixed
pressure.
Figure 5: Contour of coke formation as functions of system
pressure and CO2 adsorption.
Copyright © American Chemical Society