44317-AC4
The Effects of Aggregation on the Electronic Properties of Oligomers Designed for Organic LED's: A Stark and Microscopy Study
Organic materials have great potential for electronic and photo-physical applications such as flat-screen displays, organic transistors, and photocells. The realization of this potential relies on a more thorough understanding of the underlying photophysics, including the effects of aggregation. The photophysics of such amorphous materials are complex, since disorder leads to substantial heterogeneity in both the chromophores themselves and the coupling between them which strongly impact the spectra and fluorescence yields. This project explores these effects by studying structures that span the range from isolated chromophores to bulk, while generating a variety of data that can help clarify the underlying photophysical mechanisms.

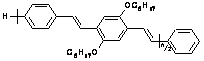 The systems under study are aggregates of oligomers of the
well-known electroluminescent polymer MEH-PPV (Fig. 1), formed as suspensions
by solvent poisoning. Structures spanning from isolated molecule to bulk are
obtained by varying: the length and substitution pattern of the oligomers or
polymer, the degree of aggregation, and the solvent. The technique of dispersed
fluorescence microscopy was implemented and applied to characterizing
individual aggregates to probe the heterogeneity in their optical properties.
Fluorescence lifetimes of both bulk and single aggregate samples were initiated
to measure the effects of aggregation on the radiative and non-radiative rates.
Electrofluorescence (EF) spectroscopy was used to measure field-induced
quenching in the oligomer and polymer systems. The results of these studies
are summarized below.
The systems under study are aggregates of oligomers of the
well-known electroluminescent polymer MEH-PPV (Fig. 1), formed as suspensions
by solvent poisoning. Structures spanning from isolated molecule to bulk are
obtained by varying: the length and substitution pattern of the oligomers or
polymer, the degree of aggregation, and the solvent. The technique of dispersed
fluorescence microscopy was implemented and applied to characterizing
individual aggregates to probe the heterogeneity in their optical properties.
Fluorescence lifetimes of both bulk and single aggregate samples were initiated
to measure the effects of aggregation on the radiative and non-radiative rates.
Electrofluorescence (EF) spectroscopy was used to measure field-induced
quenching in the oligomer and polymer systems. The results of these studies
are summarized below.
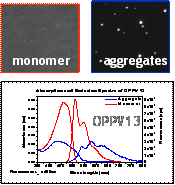
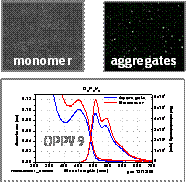
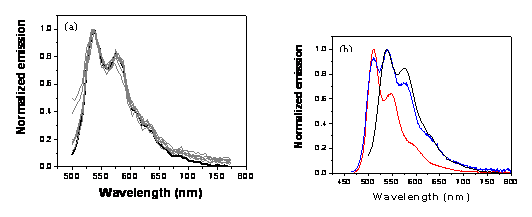
Absorption, Emission and Single Aggregate Spectroscopy: This work represents a systematic study of the effects of oligomer length, aggregate size and solvent precipitation conditions on the absorption and emission properties of PPV-like aggregates. Two representative examples are shown in Fig. 2. In the 9-ring oligomer aggregate, there is little or no change in the absorption or emission spectrum on aggregation whereas, in the 13 ring oligomer, both spectra are obviously perturbed. Moreover, the degree to which the emission spectrum is perturbed depends on aggregate size with larger aggregates showing a smaller perturbation (data not shown). Very similar intensity patterns are seen in spectra of individual oligomer aggregates (Fig. 3) indicating that the spectra are not very sensitive to the expected heterogeneity in the aggregate populations. Our current model for understanding the spectral changes on aggregation is that the longer chain oligomers form aggregates with stronger chain-chain interactions than do the smaller chain oligomers due to their stronger dispersion interactions. Interestingly, the fluorescence lifetimes of the small-chain oligomer aggregates are nearly identical to those of the corresponding monomers (data not shown) whereas, the lifetime is decreased by nearly a factor of two upon aggregation in the longer-chain species. This decrease in the emission lifetime correlates well with the factor of ~ 2 decrease in emission yield on aggregation meaning that the fluorescence quenching is due to an enhancement in the non-radiative rate.


Electric Field Effects on the Emission Properties of PPVs:
Our
group has been studying field-induced perturbations of the absorption and
emission spectra of MEH-PPV and its oligomers over the last few years. One
unexpected finding was that the applied field quenches the emission of isolated
oligomer molecules as well as the polymer when these systems are present in
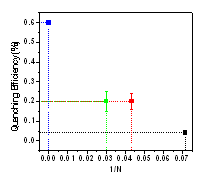
micromolar concentrations in organic glasses. The degree of fluorescence
quenching exhibits a nearly linear dependence on inverse chain length shown in
Fig. 4. The observation of emission quenching in the oligomers and in single polymer
chains was unexpected because the probability is small either of finding free
charges or of dissociating the exciton in the high dielectric environment of an
organic solvent glass. These are the two mechanisms that have been invoked to
explain field-induced emission quenching in the polymer species. In order to
understand the origin of this phenomenon a model was developed that relates the
magnitude of the quenching to the expected change in the non-radiative rate of
the molecule due to the Stark shift of the excited state in the presence of a
field. This model, which achieves quantitative agreement with the experimental
findings, presents a new paradigm for the origin of field-induced quenching in
organic semi-conductor materials at the low concentration limit. The effects
of oligomer structure and substitution pattern on the magnitude of field-induced
quenching are now being examined.