43717-B1
Porphyrin Arrays as Light Harvesting Compounds Formed from Porphyrin Modules and Metal Linkers
Our research program is focusing on the construction of porphyrin arrays built around metal centers through non–covalent interactions between the porphyrin and metals. Towards this end, we have been synthesizing porphyrins with heterocyclic substituents such an imidazole and an imidazolium substitute porphyrins as well as pyridyl porphyrins attached to osmium(II) metal centers.
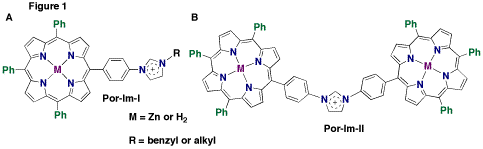
Imidazoles exhibit interesting metal binding properties, as they can bind through the nitrogen in the ring, or by forming a carbene complex. The imidazole porphyrin compounds were synthesized by two different approaches; in the first method, used for Por-Im-I, Figure 1a, the imidazole porphyrin was synthesized directly from imidazole benzaldehyde and the imidazolium was formed by the addition of the an alkyl halide or a benzyl halide. The second approach for Por-Im-II, Figure 1b, involves the synthesis of the imidazole ring between the two porphyrin rings. This compound exhibits unusual C–H hydrogen bonding as the acidity of the proton between the nitrogen atoms on the imidazole ring is enhanced by the charge on the ring.
A second project involves the formation of a series of
porphyrin-pyridyl-osmium compounds (Figure 2). The porphyrin-pyridyl-osmium
compounds have been shown the to conduct light induced electron transfer and
energy transfer reactions, depending on the presence of zinc(II) metal ions in
the porphyrin. The current project
extends this research by changing the substituents on the osmium metal ion from
bipyridine to phenanthroline and substituted bipyridines and phenanthrolines
and then studying the effect this has on the electron and energy transfer
reactions. In addition, we are
developing methods to attach the imidazole porphyrins to the osmium metal
center through the nitrogen.