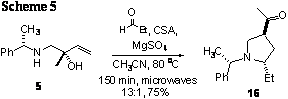42476-GB1
Development and Stereochemical Investigation of the First Microwave-Assisted Aza-Cope Rearrangement-Mannich Cyclization
As described
in previous annual reports, we have developed the first reported
microwave-assisted aza-CopeóMannich reaction [1].† This sequence generates acylpyrrolidines in a
single synthetic step while significantly reducing reaction times as compared
to analogous reactions using conventional heating, which have been Scheme 2
To
determine whether diastereoselectivity might improve at lower temperatures, the
reaction was carried out at 50 oC and at room temperature (Scheme 3, Table).† Indeed, diastereoselectivity
was modestly increased from 1.8:1 to 3.2:1 by decreasing the reaction
temperature from 80 to 50 oC, although a significantly longer
reaction time was required for complete consumption of starting material
(entries 1 and 3).† It should be noted
that, because these results are preliminary, the exact reaction time required
for complete conversion has yet to be determined.† Nonetheless, when the reaction was quenched
after 120 minutes of irradiating at 50 oC, only 11% conversion to
products was observed.† Although the
identity of the major diastereomer has yet to be rigorously identified, the
reaction presumable proceeds via chair 54
which would allow the sterically demanding phenyl group to be angled away from
the pseudo chair [5].† Subsequent Mannich
cyclization of cation 55 would lead
to acylpyrrolidine 56 as the major
isomer.†
Scheme 3
Table. Temperature
comparison of aza-CopeóMannich reactions of amino alcohol 5
entry temp (oC) time product ratioa (% yield) 1 80 30 min 1.8:1 (77) 2 50 120 min 3.0:1 (11.0)b 3 50 8 hrs 3.2 (75) 4c rt (23-25) 48 hrs 3.7 (79) bPercent of reaction mixture as determined by 1H
NMR analysis (not isolated yield) cReaction was stirred at room temperature without
microwave irradiation. We have also demonstrated
that amino alcohols 1 and 11 readily undergo microwave-assisted
aza-Cope rearrangementóMannich cyclization following condensation with
propionaldehyde (Scheme 4) [1]. †In addition, we found that by increasing the steric
demand of the amine protecting group from benzyl to benzhydryl, diastereoselectivity
for anti-2-ethyl-4-acylpyrrolidines 13 and 12 could be improved from 3:1 to 8:1, respectively.† †††††††† Surprisingly, when the analogous
aza-CopeóMannich reaction was performed on the less sterically demanding S-α-methylbenzylamino alcohol 52, a 13:1 isomeric mixture favoring the
anti-pyrrolidine 82 was recovered according to 1H and 1D nOe NMR analysis
(results obtained by Aaron Kaufmann, BS, 2009) (Scheme 19).† It is noteworthy
that, because these results are preliminary, only the relative C2-C4 stereochemistry
has been determined.† While the absolute
stereochemical determination is in progress, the indicated configuration is
based on ample precedent [5].† In
addition, due to separation issues, the stereochemistry of the minor isomer has
yet to be rigorously identified.† Both of
these determinations are the subject of ongoing work in our lab, as is the
optimization of the stereoselectivity of the aza-Cope Mannich reaction leading
to 3-acylpyrrolidine 6 (Scheme 2), and the application of diastereocontrolled
aza-Cope Mannich reactions similar to that in Scheme 5 to the synthesis of natural and unnatural alkaloids. †
References
[1] Johnson, B. F.; Marrero,
E. M.; Turley, W. A.; Lindsay, H. A. Synlett
2007, 893-896. [2] Overman, L. E.; Kakimoto,
M.-A.; Okazaki, M. E.; Meier, G. P. J.
Am. Chem. Soc. 1983, 105, 6622-6629. [3] Cooke, A.; Bennett, J.;
McDaid, E. Tetrahedron Lett. 2002, 43, 903-905. [4] Desai, H.; D'Souza, B.
R.; Johnson, B. F.; Lindsay, H. A. Synthesis
2007, 902-910. [5] (a) Agami, C.; Couty, F.;
Lin, J.; Mikaeloff, A.; Poursoulis, M.† Tetrahedron 1993, 49, 7239-7250; (b) Agami,
C.; Cases, M.; Couty, F.† J. Org. Chem. 1994, 59, 7937-7940.† (c) Agami,
C.; Couty, F.; Puchot-Kadouri, C. Synlett
1998, 449-456.†
 reported to require 10 to 72 hours [2,3].† In the simplest case, amino alcohol 48, available via epoxide aminolysis of
isoprene monoxide with benzhydrylamine [4] rapidly formed 3-acylpyrrolidine 49 when subjected to microwave
irradiation for 30 minutes at 70 oC or for 150 minutes at 50 oC
in the presence of paraformaldehyde, camphorsulfonic acid (CSA), and MgSO4
(Scheme 1). †In an effort to determine the enantiocontrol
for the aza-CopeóMannich reaction, we again used microwave-assisted epoxide aminolysis
[4] to generate amino alcohols 4 and 5 as a 1:1 mixture of diastereomers.† After chromatographic separation, we could
obtain isomerically pure amino alcohol 5
in 25% yield with an additional 70% recovery of an isomeric mixture.† The absolute stereochemistry of amino alcohol
5 was determined by X-ray
crystallographic analysis of the HBr salt.††
Unfortunately, subjecting this amino alcohol to aza-CopeóMannich
reaction conditions as before resulted in a 1.5:1 mixture of diastereomeric
acylpyrrolidines 6 (Scheme 2).†
reported to require 10 to 72 hours [2,3].† In the simplest case, amino alcohol 48, available via epoxide aminolysis of
isoprene monoxide with benzhydrylamine [4] rapidly formed 3-acylpyrrolidine 49 when subjected to microwave
irradiation for 30 minutes at 70 oC or for 150 minutes at 50 oC
in the presence of paraformaldehyde, camphorsulfonic acid (CSA), and MgSO4
(Scheme 1). †In an effort to determine the enantiocontrol
for the aza-CopeóMannich reaction, we again used microwave-assisted epoxide aminolysis
[4] to generate amino alcohols 4 and 5 as a 1:1 mixture of diastereomers.† After chromatographic separation, we could
obtain isomerically pure amino alcohol 5
in 25% yield with an additional 70% recovery of an isomeric mixture.† The absolute stereochemistry of amino alcohol
5 was determined by X-ray
crystallographic analysis of the HBr salt.††
Unfortunately, subjecting this amino alcohol to aza-CopeóMannich
reaction conditions as before resulted in a 1.5:1 mixture of diastereomeric
acylpyrrolidines 6 (Scheme 2).† 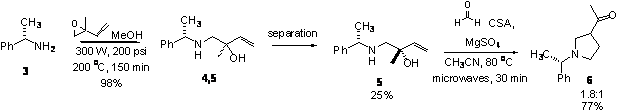
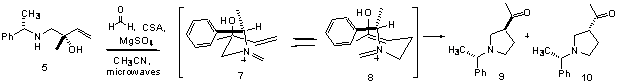
aAccording to 1H NMR analysis.