44532-G4
The Effect of Fluorine-Containing Amino Acids on Protein Secondary Structure Stability
This project focuses on determining the alpha-helix and beta-sheet propensities for various fluoro-amino acids. The long term goal of this project is to improve the stability of enzyme catalysts for applications in organic synthesis by introducing highly fluorinated amino acids. Substituting hydrocarbon amino acids with highly fluorinated amino acids can enhance protein stability, which is known as the fluoro-stabilization effect. The secondary structure propensities for the fluoro-amino acids would be useful for quantitatively predicting the effect of fluoro-amino acids on secondary structure stability to facilitate the application of enzymes in organic synthesis.
Helix Propensity We have measured the helix
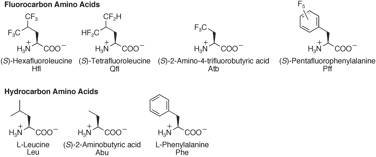
propensity of various fluoro-amino acids: (S)-5,5,5,5',5',5'-hexafluoroleucine (Hfl), (S)-5,5,5',5'-tetrafluoroleucine (Qfl), (S)-2-amino-4,4,4-trifluorobutyric acid (Atb), and (S)-pentafluorophenylalanine (Pff). The helix
propensity was determined in monomeric alanine-based peptides using circular dichroism
spectroscopy coupled with modified Lifson-Roig theory. Our results show that the helix propensity is consistently lower for the highly
fluorinated amino acids compared to the corresponding hydrocarbon amino acids
(Hfl<Qfl<Leu; Atb<Abu; Pff<Phe). To test the generality of these
results, we have also measured the helix propensity (of the same fluoro-amino
acids) in dimeric coiled coil peptides by chemical denaturation using
guanidinium chloride. Results from monomeric alanine-based peptides and dimeric
coiled coils exhibit similar trends with slightly different energetics. We have
investigated the effect of alcohol cosolvents on the monomeric alanine-based
peptides to gain further insight into the reason for the low helix propensity
of fluoro-amino acids. Based on these experiments, it appears that partial
burial of highly hydrophobic fluorocarbon side chains in the unfolded random
coil state and side chain shielding of the helix hydrogen bond in the folded
helix state both play a role in determining the helix propensity of these amino
acids. Sheet Propensity We have measured the
beta-sheet propensity for various fluoro-amino acids (Hfl, Qfl, and Pff) in the
protein G B1 domain I6A T44A double mutant. Thermal denaturation of the
proteins was monitored by circular dichroism spectroscopy. The data was
analyzed to obtain the relative beta-sheet propensity for the amino acids.
These results show a higher beta-sheet propensity for fluoro-amino acids compared the corresponding hydrocarbon amino acid
(Hfl>Qfl>Leu; Pff>Phe). Variation in
beta-sheet formation energetics for amino acids have been attributed to
hydrophobics, sterics, or both. Based on our results, sterics appears to play a
more predominant role compared to hydrophobics in determining beta-sheet propensity.
Overall, these results suggest that fluoro-amino acids should be very well
suited for stabilizing beta-sheet proteins. Future Aspects The conformational effect
upon introducing fluoro-amino acids has been investigated in this project,
suggesting that fluoro-amino acids are more suitable for stabilizing
beta-sheets compared to alpha-helices based on conformational preference. These
results forms the foundation for the next phase of this research, which will
focus on the effect of fluoro-amino acids on biological activity.