45823-AC7
Highly Functionalized Macromolecules Based on Free Radical Copolymerization of Substituted Stilbene Monomers
Synthetic macromolecules that have functional groups pendant to the polymer backbone are important polymers that enable many applications. Because of the hydrolytic stability of the carbon carbon backbone, formed in free radical polymerization of vinyl monomers, and the tolerance of free radical processes to a wide variety of functional groups, free radical polymerization is widely used to prepare functional polymers. 1,2-Disubstituted monomers offer the potential to raise the concentration of functional groups along the backbone, to likely increase the stiffness of the chain, and can yield polymers with precisely placed functionality of varying functional group densities. However most 1,2-disubstitued monomers are a challenge to polymerize. trans-Stilbene (1,2-diphenylethylene) can be readily functionalized and these functional monomers are promising unexplored monomers for use in preparing functional copolymers.
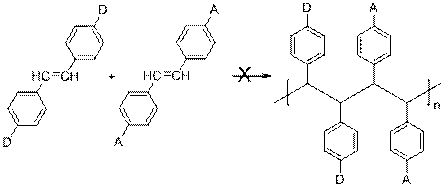
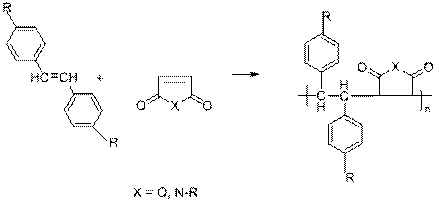
The objective of this research is to investigate the polymerization and copolymerization characteristics of substituted stilbene monomers and then study the physical properties of the resulting functional polymers that are obtained. We have prepared numerous functional stilbene monomers with donor (such as dialkyl amino) or acceptor groups (ester groups) in the 4,4'-position on the phenyl groups of stilbene. We have, as yet, been unsuccessful in polymerizing these stilbene monomers to yield a poly(phenylmethylene) backbone (Eq. 1), however we have discovered that these 4,4'-disubstituted monomers readily form alternating copolymers with maleic anhydride and N-substituted maleimides to yield new families of highly functional copolymers with unique properties (Eq. 2) (1)
Equation 1.
Equation
2.
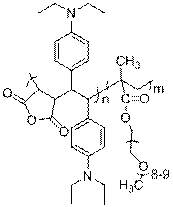
Solid state NMR studies have revealed that the copolymer of N,
N, N', N'-tetraethyl-4,4'-diaminostilbene and maleic anhydride enchains via a
cis insertion of the maleic anhydride into the growing to chain to yield an
unusual copolymer structure (I) (2). This
copolymer, upon hydrolysis, readily forms a unique polyampholyte that is that
has vicinal positive and negative charges along the backbone (II).
Double hydrophilic rod-coil block copolymers (DHBC) have
been prepared using poly(ethylene glycol methyl ether methacrylate) blocks
prepared using reversible addition-fragmentation chain transfer (RAFT)
techniques and then blocking with the N,N,N'N-tetraalkly-4,4'diaminostilbene-alt-maleic
anhydride. These unique DHBC structures
(III) show very unusual nanoparticle size growth in the presence of salt that
is attributed to a stimulated
“like-charge” attraction of polymer chains (3).
III
References:

 I
I