46291-B6
Condensed Phase Effects on the Structural Properties of Friedel-Crafts Intermediates: RF-BF3
Context: The main objective of this project is to characterize the structural properties of organofluoride – boron trifluoride complexes (RF'–BF3) in the gas-phase and in bulk, condensed-phase environments via computations and low-temperature infrared spectroscopy. These species are key intermediates in Friedel-Crafts reactions [1] - an important class of carbon-carbon forming processes that facilitate the conversion of petroleum feedstocks to commercially-viable compounds. One common example is the alkylation of benzene, viz.
The first step is the formation
of an RF–BF3 intermediate [1], a few of which have been
isolated. When R is "propyl" or larger, these intermediates produce electrolyte
solutions and are thus best described as fluoro-borate salts. However, when R
is methyl or ethyl, they are donor-acceptor type complexes that do not produce
electrolyte solutions, but still react in the same manner. In the gas-phase, CH3F–BF3
complex is quite weak with an experimental B-F' distance of 2.42ü [2]. Thus,
its solution-phase reactivity can only be rationalized by invoking a
substantial structural re-arrangement that results from interactions with the
solvent. The main goal of this work is to characterize this transition from
"weak donor-acceptor complex" to "effective carbo-cation", as a function of
both internal effects (e.g. changes in the R substituent), and environmental
factors (i.e. polarity of the solvent).
Infrastructure: A
substantial portion of the proposed work involves computations, not only for
predictions of gas-phase structures and frequencies, but also energy-profiles
along the B-F' distance coordinate in both the gas-phase and in bulk,
dielectric media (via continuum solvation models). Thus, the initial efforts
were focused on obtaining a 6- or 8-processor computational cluster with a
queuing system to maximize the efficiency of the system. After the acquisition
of this grant, however, an opportunity to pool resources with a colleague and
pursue a larger system became available, and furthermore, personnel in UWEC's Learning
Technology Services (LTS) unit became
active collaborators interested in overseeing the development and
implementation of such a system. For the past 6-months, we have been running
computational jobs on a 32-processor test system, using software purchased
through these pooled funds. Some of these were designed simply to provide
performance benchmarks, others produced the results shown below. Nonetheless,
this effort has enabled LTS personnel to assess the CPU, memory, and storage
needs for various types of computations, as to obtain the maximum possible
benefit from our investment. In the future, this collaboration may lead to a
coordinated effort that involves the creation of a center that involves several
departments. In the next few weeks, we will be purchasing our first installment
of high-performance computing hardware, a 32 or 40-processor cluster, which
will enable very high-level computations to be performed locally.
Results: HF–BF3:
Validating DFT methods on a small test system with a known structure The HF–BF3
complex, though it lacks the R group necessary to participate in a reaction
such as (1), is an ideal system upon which to validate computational methods
since it is the smallest member of the RF'–BF3 class of complexes,
and moreover, an experimental structure is available. This system is the
functional equivalent of fluoroboric acid "HBF4", but the
experimental structure of this system has the HF weakly coordinated to a
largely-undistorted BF3, with an intermolecular B-F' distance of
2.54 ü [2]. Awe found that X3LYP performed the best among 6 various DFT methods
and MP2 (with the aug-cc-pVTZ basis set),not only interms of agreement between
the experimental and theoretical structures, but also the accuracy of predicting
gas-phase BF3 frequencies. The calculated B-F' distance via this
method is 2.535 ü, and the binding energy is 2.9 kcal/mol.
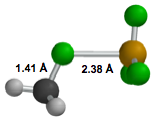
CH3F–BF3:
We subsequently
calculated X3LYP/aug-cc-pVTZ structures for four possible Cs-symmetry
conformations of CH3F–BF3. The minimum energy
conformer is shown below, and has no imaginary vibrational frequencies, in
contrast to the other three. The calculated B-F distance (2.41ü) compares quite
favorably with the preliminary experimental result (2.42 ü) [2], and the
binding energy is predicted to be 3.6 kcal/mol. Thus, the substitution of a
methyl group for the hydrogen causes a notable contraction of the B-F' distance
(> 0.1ü ) and a 0.6 kcal/mol (20%) increase in the binding energy. At this
point we have also obtained a preliminary prediction of the structural changes
that occur in chloroform solution. Surprisingly, the equilibrium B-F' distance
is predicted to contract by only about 0.03ü,
and the C-F' distance only increases by about 0.01ü. A more detailed
assessment is in progress.
GAS PHASE
CHLOROFORM
SoLUTION
References: 1. Olah, G.A. Friedel-Crafts and Related Reactions; Wiley/Interscience: New York, 1963. 2. Leopold, K.R.; Canagaratna, M.; Phillips, J.A. Accts.
Chem. Res. 1997, 30, 57.
 + RF'
+ BF3
+ RF'
+ BF3

 + HF' +
BF3 (1)
+ HF' +
BF3 (1) The
smallest, yet possibly most interesting intermediate
The
smallest, yet possibly most interesting intermediate