

44908-GB1
Building Enantioselective Ligands: New Frontiers with Oxazolines
Our research group is excited to report that we have x-ray structural proof to confirm that we have made the first chiral bis(oxazoline)-isoindoline ligand as a metal complex. A summary of the progress is outlined below. Progress for Year 2 was slowed by the transfer of my lab to a new institution however, the lab was reestablished in the spring of 2008 and I had 2 undergraduate student researchers recruited for summer research on this project. The early part of the summer was spent training and reestablishing our synthetic protocols to prepare the precursors (synthons) of 50; chiral 1-(H)(Ph) and 1-(H)(iPr). Several trial coupling reactions between the synthons and 3, 4, or the 1,3-diimido-4 derivative were tested in order to try and obtain the proposed 50-(H)(Ph) and 50-(H)(iPr) ligands. All attempts to follow literature procedures that condensed ortho-aminopyridine (or liquid amine compounds) to 3, 4, or the 1,3-diimido-4 derivative were unsuccessful as they use high temperature conditions for prolonged periods of time or require the amine to be a liquid for lower temperature reactions. The former conditions are too harsh for the synthons as they are observed decomposing within hours of reaction. Attempts to form the ligands using microwave synthetic techniques (high temperatures but very short reaction times) also failed, with numerous decomposition byproducts being formed.
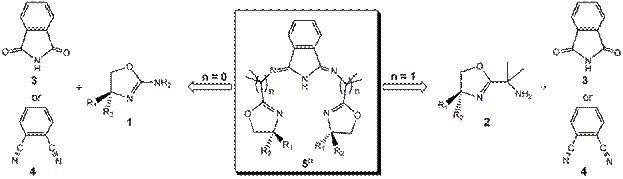
The key was to use less severe conditions based on the protocol reported for the
formation of 1,2-bis(oxazole)benzene where ZnCl2
was a catalyst. We reported in Year 1 that we isolated an air-stable
bright yellow (50-(H)(Ph))ZnIICl (6) complex based on 1H
NMR analysis, atomic absorption spectrometry (confirmed presence of zinc), and electrospray
mass spectrometry (parent ion peak that corresponds to the [Zn(50-(H)(Ph))]+
fragment) using this protocol. We spent
much of our past summer using this low yield protocol to re-synthesize and
isolate more of the compound and we were fortunate enough to grow x-ray quality
single crystals. Through a collaboration
with the x-ray lab at the
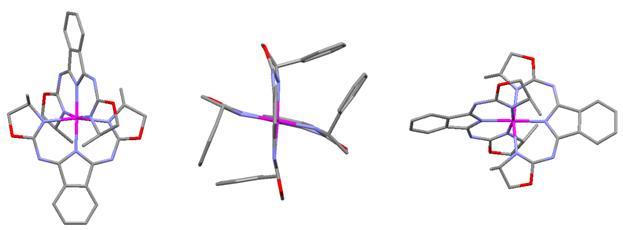
All of the current research (Year 2) was performed by two undergraduate students at the University of San Diego over the summer of 2008. Jessica Cryder worked on both the 50 and 51 system from June 2008 to the end of August 2008 and was the student who performed the bulk of the synthetic protocol testing (including the microwave reactions). Ryan Haywood worked to help isolate the Zn[(50-(H)(Ph)]2 complex from June 2008 to August 2008. Together they were able to isolate and characterize this first chiral bis(oxazoline)-isoindoline complex in solution and have x-ray crystallographic analysis provide the solid state structural proof of the complex. Jessica presented the work at the weekly departmental summer research group meeting. Both students are continuing in my group: Ryan until January (graduates with B.A. Chemistry), and Jessica throughout the 2008-2009 academic year.