46727-G10
Solute Morphology And Transport In Polymeric Fuel Cell Materials
The Madsen lab has been exploring two areas of research within this PRF-G project: 1) molecular alignment in ionomers such as Nafion ® and sulfonated polysulfones, and 2) diffusion of water and other solutes absorbed into such ionomers. These studies have produced new morphological models for ionomer membranes, and have revealed new ways to optimize proton, ion, and small molecule transport in these materials. Such information will allow for greatly improved efficiency and cost of hydrogen fuel cells, lightweight batteries, mechanical actuators, and water desalinization systems.
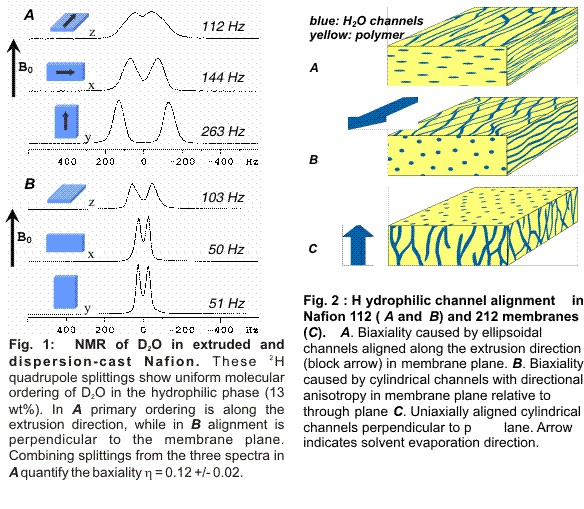
Area (1) has yielded dramatic results (see Figs. 1 and 2) regarding alignment of the hydrophilic channels in Nafion membranes processed under different conditions. We have published one paper in Macromolecules and another is under review. We have used NMR quadrupole splittings on D2O absorbed into Nafion membranes (Fig. 1) to measure orientational order parameters to quantify molecular alignment. We measure uniform alignment in extruded membranes, and can conveniently quantify the biaxiality parameter. Surprisingly, dispersion-cast Nafion exhibits alignment through the membrane plane, which may produce higher proton or other ion conductivity and thus is desirable for many device applications. Fig. 2 depicts our models for the alignment modes consistent with our NMR data. Using these measurement techniques in conjunction with enhanced processing methods, e.g., casting in a magnetic field, promises to enhance ionomer device performance.
Area (2) has involved close
collaboration with Profs. McGrath, Long, and
Measuring self-diffusion coefficients D using pulsed-field-gradient (PFG) NMR yields quantitative information regarding the transport of small molecules or ions in membranes. The chemical specificity of NMR allows us to determine D for separate components, e. g., IL cations and anions and co-diluents such as water. A critical factor for operation of ionomer-based artificial muscle actuators is that of water uptake influencing actuator performance. Our methods precisely measure the wt % uptakes of each component relative to the membrane using NMR signal intensities. Thus, we can quantify the water absorbed at ambient (or any other) humidity levels, and correlate that with D as well as with fuel cell proton conductivity or mechanical actuator response. In our measurements on ionic polymer actuators, water is absorbed in a 1:1 mol ratio with the IL, which produces faster dynamics in both the water and the IL.
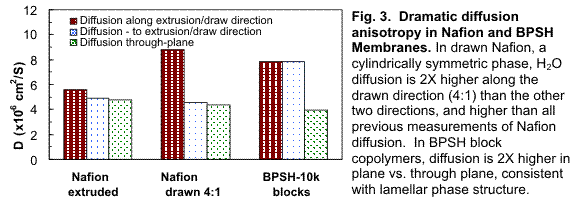
We have also probed the anisotropy of diffusion using multi-axis PFG-NMR, and correlated this information with our alignment (spectroscopy) measurements. Fig. 3 shows clear diffusion anisotropy in the extruded membrane of Figs. 1 and 2, and in drawn Nafion and oriented sulfonated biphenyl sulfone (BPSH) block copolymers. Nafion exhibits a cylindrically-symmetric channel morphology, while BPSH is a lamellar (planar symmetry) phase. Our next step is to build an apparatus to cast membranes inside the NMR spectrometer in an attempt to orient these materials using the magnetic field while observing development of anisotropy in situ.
This work has resulted in 9 presentations during this reporting period, a publication in Macromolecules, and a paper under review in Macromolecules. We are preparing to submit another manuscript involving ionic liquid and water diffusion. We expect this work to have far reaching implications for polymeric fuel cells, mechanical actuators, and battery designs.