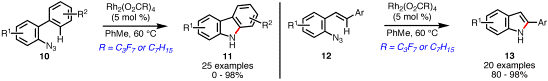46850-G1
Development of New Methodologies Involving 2H-azirines
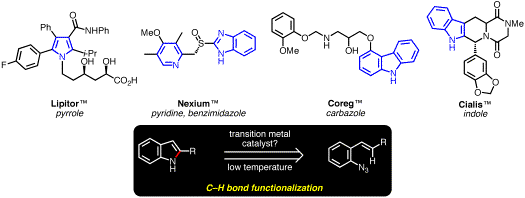
We have discovered that N-heterocycles can be synthesized through the functionalization of C–H bonds from the transition metal-catalyzed decomposition of readily accessible vinyl- and aryl azides. This report summarizes our research progress that has culminated in the synthesis of indoles, pyrroles, carbazoles, and benzimidazoles. These N-heterocyclic motifs are found in a variety of pharmaceuticals, materials, and natural products (Scheme 1).
Scheme 1. Our Approach to N-Heterocyclic Motifs Present in Important Pharmaceuticals. Rhodium(II)-Catalyzed Transformation of Azidoacrylates. As part of a program aimed at the
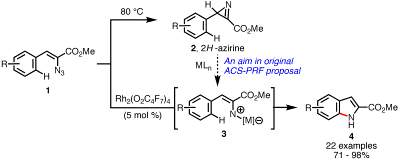
discovery of new metal-mediated reactions involving 2H-azirines (2), we discovered that vinyl azides (1), progenitors of azirines, could be
decomposed in the presence of dirhodium(II) carboxylates to afford a range of
indoles in high yields (Scheme 2).
We decided to use the PRF Type G grant to support our investigations
into this area during the budget period of 8/2007 – 8/2008. Our efforts during this period
culminated in three communications in top journals (Angewandte Chemie
International Edition
and Organic Letters) as well as one full paper, which was submitted to Journal of the
American Chemical Society. The results we generated
during this time period were instrumental in securing support from the National
Institutes of Health to develop new methodology involving the transition
metal-catalyzed decomposition of azides (NIGMS R01GM084945).
Scheme 2. Rhodium-catalyzed Synthesis of Indoles. Transition Metal-Catalyzed
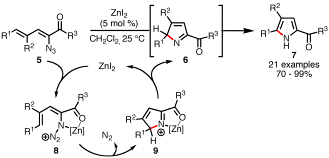
Generation of Pyrroles from Dienyl Azides. We
found that our initial results could be extended to create pyrroles 7 from dienyl azides 5 (Scheme 3). In contrast to our previous study,
which identified rhodium(II) perfluorobutyrate as the only efficient catalyst
for indole formation, we found that a range of metal salts (Rh2(O2CC3F7)4,
Cu(OTf)2, FeBr2, and ZnI2) smoothly catalyzed
the generation of 7 at room temperature. Zinc
iodide was found to be the most reactive catalyst and produced a range of di-
and trisubstituted pyrroles from 5.
Qualitatively, we observed that electron rich dienyl azides reacted much
more rapidly than the corresponding electron poor substrates. These reactivity trends and the
increased number of capable transition metal catalysts led us to suggest that
the mechanism involves Lewis acid activation of the azide (to give 8) instead of nitrogen atom transfer.
Scheme 3. ZnI2-Catalyzed Synthesis of Pyrroles. Rhodium(II)-Catalyzed
Decomposition of Aryl Azides.
Rhodium-mediated N-heterocycle formation is not limited to azidoacrylate
starting materials: submission of 2-azidobiaryls 10 to catalytic amounts of rhodium(II)
perfluorobutyrate (or octanoate) affords carbazoles 11 (Scheme 4). In contrast to dienyl azides, the
reactivity of 10
mirrored that of azidoacrylate 1: no product was formed in the presence of ZnI2
or Cu(OTf)2. The
reaction was tolerant of electron-donating and electron-withdrawing groups on
both aryl portions of 10. In addition to
carbazoles, 2-substituted indoles 13 can be readily formed from azidostilbenes 12 using catalytic quantities of
rhodium(II) carboxylates. In
contrast to the behavior of 2-azidobiaryls 10, the reactivity of 12 was enhanced with both
electron-rich and electron-poor R1-substituents.
Scheme 4. Rhodium-Catalyzed Synthesis of Carbazoles and Indoles from
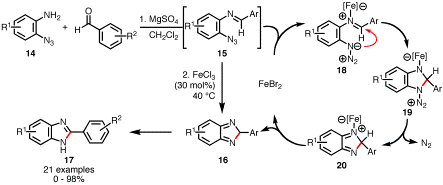
Aryl Azides. Fe(II)-Catalyzed N-Heterocycle Formation. The identity of the ortho-substituent of an aryl azide exerts
a powerful effect on its reactivity towards transition metals. Substitution the vinyl group with an
imine disables a rhodium-catalyzed process. After extensive scanning of Lewis acidic transition metals,
we found that iron(II) bromide facilitates benzimidazole 17 formation from in situ generated
aryl imines 15 (Scheme
5). Therefore in one operation,
2-azidoanilines can be transformed into benzimidazoles. We believe that a Lewis acid mechanism
accounts for product formation.
Scheme 5. Fe(II)-Catalyzed Benzimidazole Formation Through a Lewis
Acidic Mechanism. In conclusion, we have found that
transition metals can catalyze the transformation of aryl- and vinyl C–H
bonds into C–N bonds using tethered azides as the nitrogen atom
source. The support of ACS-PRF
enabled us to generate the requisite results to secure longer term (9/2008
– 9/2013) funding from the National Institutes of Health to continue to
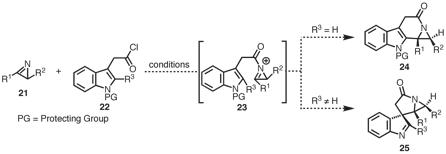
study the reactivity of azides toward transition metals. For the next budget period 8/2008
– 8/2009, we will return to an original aim of our PRF proposal and study
the ambiphilic nature of 2H-azirines. We
anticipate that exposure of azirines 21 to reagents, such as acid chloride 22, will trigger a cascade process to
form N-heterocycles
such as 24 or 25 in one synthetic operation (Scheme
6).
Scheme 6. Future Plans: Exploit the Ambiphilic Nature of 2H-Azirines.