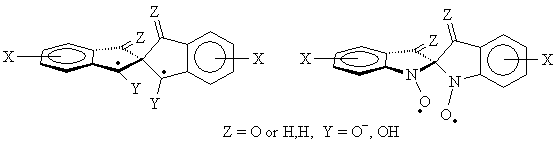45651-AC4
Novel Topologies for Unpaired-Electron Interactions
| We are preparing di- and tri-radicals with new topologies of intramolecular and intermolecular spin interactions. In one category of
radicals, the new species are designed by combining two identical radical
subunits in such a way as to allow for their singly-occupied molecular orbitals
to spiroconjugate, giving two new molecular orbitals spanning the entire
molecule. The new topology yields species with electrons delocalized in two
perpendicular planes. The new species under investigation include spiroconjugated
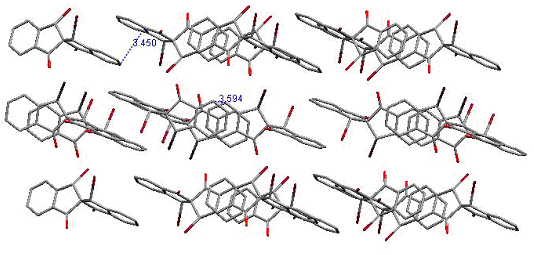
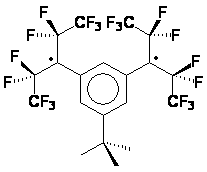
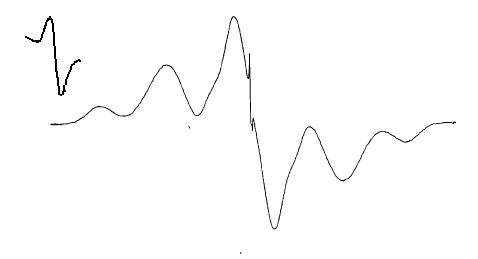
radicals shown below. In a way, these diradicals are intermediate between the non-Kekulé diradicals (the radical sites can bond to give the corresponding cyclopropanes only if there are significant distortions to the spiro skeleton), and 4n annulenes with which they share the property of complete spatial orbital coextension, if the spiroconjugation is fully “turned on”. On the other hand, if the spiroconjugation is “turned off”, these radicals are analogous to the well-studied localized 1,3-diyls, except for their perpendicular geometry. Since the two orbitals holding the unpaired electrons are not degenerate, there exists a possibility for both unpaired electrons to end up in the lower of the two spiroconjugated orbitals. A singlet state of that kind would correspond to a Kekulé structure (cyclopropane, possible only with significant distortions and not an energy minimum for any of the structures included here as found by ab initio calculations, or to a non-Kekulé “perpendicular” singlet, that, perhaps, may be described as by two ionic resonance structures with alternating positive/negative charges on the benzylic centers. The tendency for such electron pairing would increase with the spiroconjugation strength because of the increase in the energy separation of the two frontier orbitals. Understanding of that balance of interactions, and how it is affected by the substituents, should lead to tunable diradicals. In that context we were able to observe that one of our radical precursors (Z = O, Y = H,Br) had a crystal packing that allows for π-π interactions in two dimensions. The two halves of the spiro molecule participate in formation of two independent π stacks with close separations between aromatic rings. With the spiroconjugation turned on (in the diradicals) such an arrangement would yield electronically coupled stacks in two perpendicular dimension, increasing dimensionality of the interactions, and allowing for many alternative electronic paths for spin-spin interactions. Such materials should be much more robust magnets if ferromagnetic ordering could be achieved. To our knowledge, this is a first example of such a potential 3D interaction network in organic crystals Second category involves tertiary radicals with large perfluoroalkyl groups. The perfluoroalkyl groups furnish steric barriers to the undesired bond-forming reactions, and provide the opportunity to explore intramolecular spin-spin interactions between orbitals of parallel and perpendicular topologies. The radicals are constructed in such a way as to have their radical centers buried inside and have perfluorinated alkyl groups exposed to the outside - not unlike molecular "teflon balls". The new species under exploration include the "perfluoro" diiradical shown below. The diradical was produced in frozen solution (glass) by irradiation of the corresponding bromide.. Significant concentrations of this species could be obtained in this way, as attested by the observation of the half-field transition which is predicted to be very weak for diradicals with this geometry.. The ESR spectrum of the diradical is shown below (Figure 1). The zero field parameters (D = 0.0144 cm-1 and E < 0.001 cm-1) are very similar to those found is the simple m-xylylene diradical (D = 0.011 cm-1, E < 0.001 cm-1). These observations are consistent with the tiplet diradical, and the triplet ground state have been indicated for this species by the Curie-law studies on the Δm = 2 transition in the 200 - 77 K range (studies down to 4 K are underway).
|