

45927-AC9
Measurement of Molecular and Thermal Diffusion Coeffcients in Model Petroleum Fluids
The progress on the project was made in two directions. One related to theory and measurements using he thermogravtational column and other related to the beam deflection apparatus. The project allowed Aalna Leahy-Dios to complete her PhD at Yale Univers ity. Based on this project we published two papers, one in AIChE J. and the other in J. Physical Chemistry B. The AIChE J. paper allows for the first time to compute molecular diffusion coefficients for multicomponent mixtures which are key parameters of CO2 injection in fractured hydrocarbon reservoirs.
In the following we briefly discuss the theoretical work and measurements using the thermogratvitational column first and then report the progress on the beam deflection apparatus.
Unified Model for Molecular Diffusion Coefficients in Multicomponent Mixtures
We have developed a unified model for the calculation of diffusion coefficients of gas, liquid and supercritical states of non-polar multicomponent mixtures. A new approach is proposed for the binary infinite dilution diffusion coefficients. The generalized Vignes relation is used in multicomponent mixtures. Non-ideality is rigorously described by the fugacity derivatives evaluated by the volume-translated Peng-Robinson equation of state. Predictions for highly non-ideal gas and liquid multicomponent mixtures demonstrate the reliability of the proposed methodology.
New Thermal Diffusion Coefficients for Binary Hydrocarbon Mixtures
New thermal diffusion coefficients of binary mixtures are measured for n-decane–n-alkanes, and 1-methylnaphthalene–n-alkanes, with 25 wt% and 75 wt%, at 25 ºC and 1 atm, using the thermogravitational column technique. The alkanes range from n-pentane to n-eicosane. The new results confirm the recently observed non-monotonic behavior of thermal diffusion coefficients with molecular weight, for binary mixtures of n-decane–n-alkanes, at the compositions studied. In this work, the mobility and similarity effects on thermal diffusion coefficients are quantified for binary mixtures. Based on a new simple analysis, we show that the thermal diffusion coefficient of a binary mixture has a finite value at the critical point, unlike the molecular diffusion coefficient. We also show that the thermal diffusion coefficients and the viscosity of the binary mixtures are closely related.
Beam Deflection Apparatus for Measuring Binary and Ternary Molecular and Thermal Diffusion Coefficients
The Beam Deflection Apparatus was constructed and initially used by Prof. Sengers and co-workers at The University of Maryland during the 1990s. It was donated to Yale University and was transferred to Yale after the announcement of the award. In the first step an optical table was ordered to assemble the apparatus in a stable environment with minimum mechanical vibrations and luminous interferences. A desktop and the software Labview® were purchased for data acquisition. A new laser(He-Ne Laser ) was also purchased . A 15- in long precision level with a precision of 0.04 millimeters per meter was also purchased. The optical table was leveled to a tilt angle of 10-seconds. Figure 1 shows the setup.
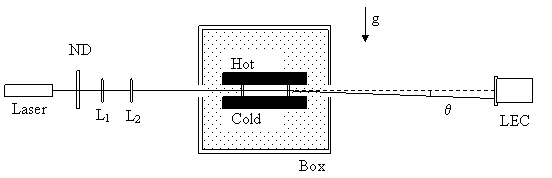
Figure 1: Schematic representation of the apparatus.
We then mounted the cell on a new adjustable multi-axis tilt platform with acrylic plastic sheet to reduce heat loss through the mounting base and checked and fixed the cell level.
The apparatus from the University of Maryland had 8 thermistors epoxied to the cell. In order to have a better understanding of experimental setup dependence on external parameters, 7 new thermistors were installed. The horizontal temperature difference within the cell plate was measured to be 0.01oC. We were then ready to examine laser stability.
Laser Stability- By performance of temperature stability tests we found that the the laser beam position is very dependent on room temperature variation. Figure 2 and 3 show the strong dependency of the water bath temperature and the laser stability.
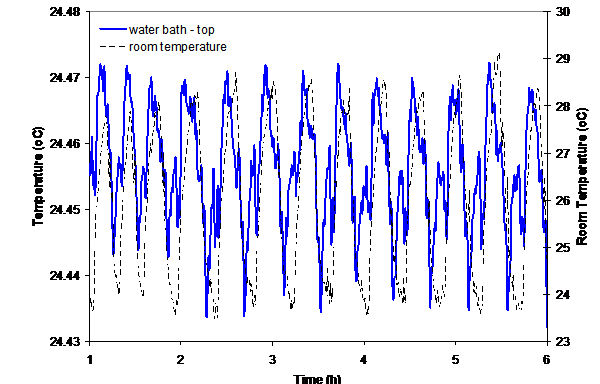
Figure 2: Temperature variation in room and water baths.
A room temperature variation of 5 ºC causes a maximum temperature variation of 0.04 ºC in the water baths. Within one cycle of room temperature variation (lasting 20 minutes), the temperature variation of the water baths was around 0.02 ºC. If the room temperature were kept constant, the temperature control of the water baths would most likely be acceptable.
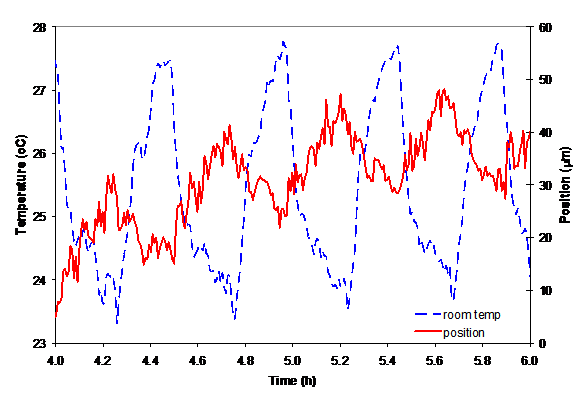
Figure 3: Room temperature and laser beam position variations.
In Fig. 3 we show the laser beam position variation when the beam passes through the empty cell. There are two different oscillation frequencies in the laser beam position signal. The lower frequency of the laser beam oscillation is related to room temperature variations. The cause for the high frequency in the laser position variation could be related to the finer temperature variations inside the acrylic box.
Various measurements show that we would need a temperature control with greater temperature stability and a better room temperature control. We have ordered and installed an in-plant office and are in the process of setting up a temperature control unit and are hopeful that with this last step we can begin the measurements.