
Back to Table of Contents
46157-G1
Chiral Acyclic Carbene Ligands for the Asymmetric Suzuki Coupling Reaction
Sukwon Hong, University of Florida
I. Result Summary
1.1.
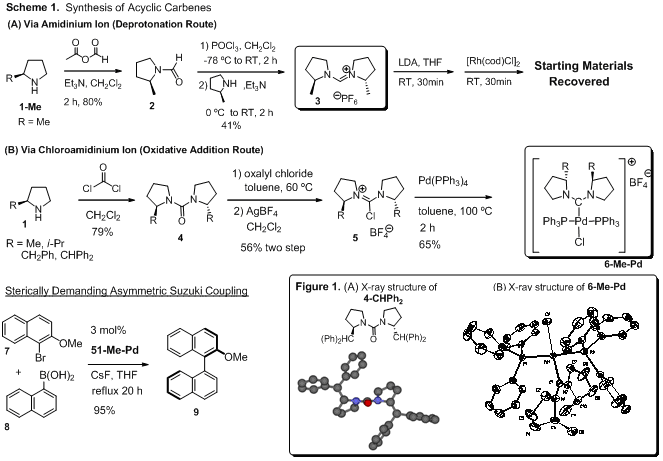
Synthesis of Acyclic Carbene-Pd complexes. The novel electron rich acyclic diaminocarbenes (ADC) based
on C2-symmetric azacycles
were designed to create a chiral environment close to the metal center. We
first explored synthetic routes to these acyclic carbene architectures using
2-substituted pyrrolidines (1)
either commercially available (R = Me, CHPh2) or prepared from
reduction of the 5-alkylpyrrolidin-2-one compounds (R= i-Pr, CH2Ph)
(Scheme 1). Deprotonation of the formamidinium ion (3) with LDA was unsuccessful although we didn't extensively screen
different bases or reaction conditions. On the other hand, oxidative addition
of the chloroamidinium ion (5) by
Pd(PPh3)4
proceeded smoothly to form the chiral ADC Pd complex, [(ADC)Pd(PPh3)2Cl]+BF4-
(6-Me-Pd) which was characterized by
X-ray crystallography (Figure 1B). Note that X-ray structures of ureas show
alkyl substituents at the 2-positions are located at the proximal side to
carbonyl (Figure 1A), however, when complexed with Pd(PPh3)4,
the alkyl substituents moved to the distal side to carbonyl apparently reducing
steric interactions with PPh3 ligands. The ADC-Pd complex is an active catalyst for
sterically demanding asymmetric Suzuki coupling reactions, however, observed
enantiomeric excesses were low (5-7% ee) presumably due to the conformational
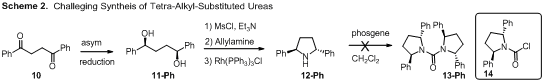
flexibility.C2-symmetric trans-2,5-diphenylpyrrolidine
(12-Ph) was prepared by the known
methods. Attempts at synthesis of a tetra-alkyl-substituted urea (13-Ph) using phosgene were unsuccessful,
affording a surprisingly stable carbamoyl chloride compound (14) instead. The
tetra-alkyl-substituted urea formation seems to be sensitive to steric demands.
1.2.
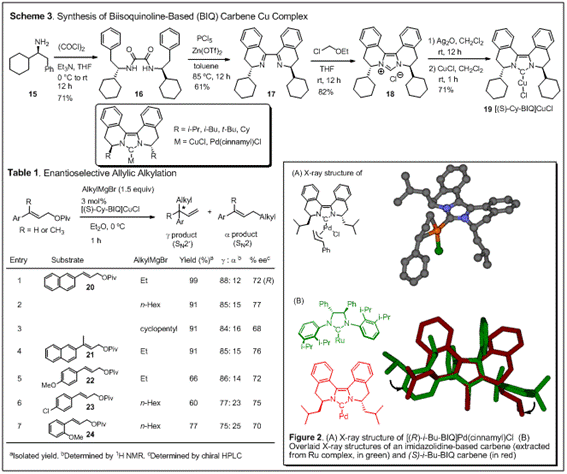
Biisoquinoline-based chiral diaminocarbene ligands. We have
synthesized several biisoquinoline-based tricyclic chiral diaminocarbene
ligands (BIQ) and their Pd and Cu complexes. The chiral environment is created
in close proximity to the metal center, which is confirmed by an X-ray crystal
structure (Figure 2). This feature can be clearly seen from the overlaid X-ray
structures of an imidazolidine-based carbene ligand and the BIQ ligand (Figure
2B). The concise ligand synthesis is highlighted by a modified
Bischler-Napieralski cyclization of bisamides (16) prepared from readily available chiral phenethylamines (15), and allows easy variation of the
stereodifferentiating groups (Scheme 3). After a brief survey of reaction
conditions and ligand structures, it was found that the cyclohexyl-BIQ-copper
complex is an efficient catalyst for enantioselective SN2' allylic
alkylation with Grignard reagents, showing SN2' regioselectivity
higher than 5:1 and enantioselectivity in the range of 68-77% ee (Table 1). In
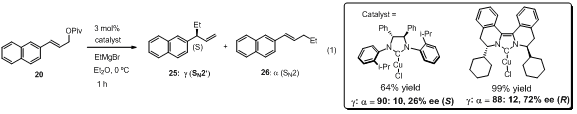
addition, when the dihydroimidazolium-based chiral NHC ligand was tested under
the same reaction conditions, similarly high SN2' regioselectivity
(90:10) was obtained but the observed enantioselectivity (26% ee) was lower
than those with BIQ ligands (eq. 1).
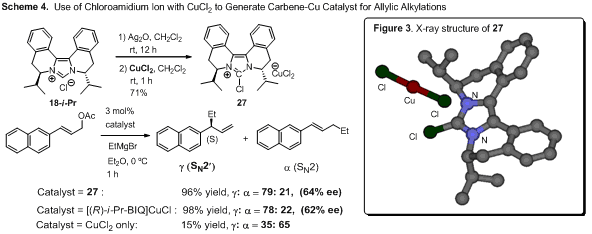
1.3. In situ generation of the Cu-carbene
catalyst from a chloroamidinium ion and CuCl. While we were
screening the reaction conditions, we found that CuCl2 gave almost
identical results compared to CuCl (Scheme 4). Rather surprisingly, the X-ray
structure obtained from a single crystal of CuCl2 complex was not
the carbene-Cu(II) complex but an ion pair of the chloroimidazolium cation and
[CuCl2]- anion (27)
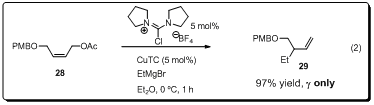
(Figure 3). When chloroamidinium ions were mixed with CuCl2 (or CuCl
or CuTC) and used in the allylic alkylation reaction of Grignard reagent,
allylic alkylation products were obtained in high yields with excellent SN2′
regioselectivity, suggesting that presumably the active Cu-carbene catalyst
might be generated in situ from the chloroamidinium salt and CuCl2
(or CuCl) in the presence of Grignard reagent (eq. 2). It is important to note
that the acyclic carbene-Cu complexes
can be easily generated by this route and the chloroamidinium ions can be
prepared from the corresponding ureas. Currently, we are studying asymmetric
catalysis by chiral acyclic carbene-Cu complexes prepared through
this in-situ generation route.
II. Impact on the Principal Investigator's Career
The ACS PRF
grant has been very helpful to initiate the proposed research project. This
grant made possible a research assistantship for one graduate student (David R.
Snead) during the summer of 2007 and resulted in one publication and
three other manuscripts in various stages of publications. Thanks to the early
support by ACS-PRF, we obtained important preliminary results, and this
research is evolved into other research program that is now funded by Florida
Department of Health (James & Esther King Biomedical Research Program) for
the next three years.
Back to top









