

45738-G7
Unusual Aromaticity and Organic Semiconductor Performance
Research conducted over the past funding period has been involved with two of the three major avenues described in the initial proposal: exploration of methano[10]annulene and borepin-based organic electronic materials. This research has resulted in two publications, with two more set for submission in the very near future.
In work with the annulene-based materials, we verified our initial design hypothesis by demonstrating that annulenes facilitate greater charge delocalization relative to comparable materials built from more benzenoid aromatics (in the current case, naphthalene). This was determined through synthesis of electropolymerizable monomers and subsequent characterization of their polymer products. The annulene polymer derived from 1 displayed much more reversible electrochemistry reminiscent of other thiophene-based conducting polymers. In contrast, within naphthalene materials derived from monomer 2, we observed irreversible electrochemical kinetics associated with material that poses a barrier to electron transfer at the electrode interface. We also found the naphthalene materials under spectroelectrochemical analysis displayed a marked isosbestic point during the transition from the insulating neutral aromatic electronic structure to the presumably conductive and delocalized quinoidal structure. This isosbestic point suggests that the naphthyl unit acts as an energetic barrier to prevent delocalization of carriers relative to the more "diffuse" annulene, where a broad absorption profile is observed upon oxidation.
Figure 1: Bithiophene-based monomers 1 and 2 (left), polymer CV (a,b) and spectroelectrochemistry (c,d).
|
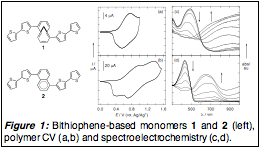
Figure 2: Furan-based annulene polymer spectroelectrochemistry |
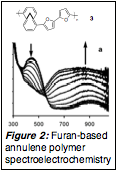
We extended this general concept to the synthesis of furan-based conjugated polymers, although not exactly in the spirit detailed in the grant application. Furan is of interest as a monomer derived from biomass, yet its use for electronic materials has been curtailed due to its very high oxidation potential, one that often is sufficient to trigger decomposition rather than clean polymerization. We reasoned that the established cation-stabilizing abilities of the annulene core should render the electropolymerization intermediates associated with furan-appended annulenes more stable when compared to bare furan, but that they should be reactive enough to polymerize. We demonstrated how the annulene lowered the monomer oxidation potential and offered better electrochromic properties in the resulting polymer 3. We have completed a detailed optical, electrochemical and theoretical joint study of 3 and related systems in order to evaluate the impacts that the annulene core bring to the bulk electrical properties. We are waiting for final in situ electrical conductivity data prior to submitting this work for publication.
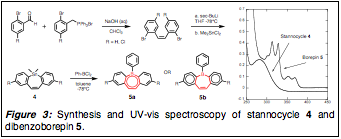
Our work with boron-based aromatics involved the synthesis of an as-yet unknown aromatic system that could be functionalized into complex polymeric materials. Although we needed to alter our proposed synthetic strategy substantially, a Wittig-type olefination provided a stilbene precursor that readily underwent stannocyclization to 4 followed by tin-boron exchange to yield the borepin 5 (depicted in Figure 3 as two possible electronic resonance contributors). Unfortunately, environmental sensitivity proved problematic, and the B-phenyl derivative decomposed within minutes thereby requiring handling in inert atmosphere. As this would preclude any purification of chemically elaborated products, we altered the aryl boron group to provide more steric bulk; although standard mesityl (Mes, 2,4,6-trimethylphenyl) was ineffective at protecting the boron,
Figure 3: Synthesis and UV-vis spectroscopy of stannocycle 4 and dibenzoborepin 5.
|
 we found the more demanding Mes* (2,4,6-tri-t-butylphenyl) allowed
us to manipulate and even chromatograph the resulting borepin with no special
safeguards! The aryl halides have
so far resisted cross-coupling, and our work is ongoing. We are also exploring
the electronic fine-tuning through addressing the vacant boron p orbital with
Lewis bases such as fluoride anion.
We are confident that in the coming weeks we will have new borepins
bearing extended p-conjugation due to the
incorporation of unsaturated groups.
we found the more demanding Mes* (2,4,6-tri-t-butylphenyl) allowed
us to manipulate and even chromatograph the resulting borepin with no special
safeguards! The aryl halides have
so far resisted cross-coupling, and our work is ongoing. We are also exploring
the electronic fine-tuning through addressing the vacant boron p orbital with
Lewis bases such as fluoride anion.
We are confident that in the coming weeks we will have new borepins
bearing extended p-conjugation due to the
incorporation of unsaturated groups. Support from the PRF has enabled our young group to generate initial results of sufficient caliber for publication. This early research is expected to make future grant applications more competitive. Students have been exposed to synthetic chemistry of aromatic and pi-conjugated systems as well as gained familiarity with electrochemical and optical characterization methods, all important skill sets for their future careers. Work in progress includes the following: (1) exploration of chemically synthesized annulene polymers for fluorescence studies and for semiconductor mobility measurements (as opposed to electrode-bound electrochemical synthesis of analytical quantities of material); (2) continued investigation of random copolymers using commercial thiophene monomers and our annulene monomers as studies with in situ conductivity and spectroelectrochemistry; (3) development of suitable chemistry to functionalize the halogen-substituted borepin rings for incorporation into more complex molecular and polymeric materials.