44568-B5
Electrodeposition of Metal Alloy and Mixed Oxide Films Using a Single-Precursor Tetranuclear Heteropolymetallic Complexes
In our first report, cyclic
voltammetry (CV) studies have shown that Cu3Co complex is
electrochemically active and at higher cathodic potential the complex forms a
deposit on the electrode surface. In this work, we have extended the CV
studies to Cu2Co2 and CuCo3, and the results
demonstrate similar electrochemical trends for both complexes. Analogous
electrochemical results were also obtained using hydrodynamic rotating disk
electrode (RDE) volatmmetry for Cu3Co and Cu2Co2
complexes. RDE voltammetry experiment of CuCo3 was not performed
due to a very limited amount of sample obtained from the synthesis. We also
conducted scanning electron microscopy (SEM)/energy dispersive x-ray
spectroscopy (EDS) studies for an electrodeposited film acquired from Cu3Co
complex.
All the heteropolymetallic complexes were
synthesized in methylene chloride by means of transmetallation reaction and the
by product of the reaction, Cu(NS)2, was separated from the
complexes by gel permeation chromatography. 1-2
The reactants used for the transmetallation reactions were synthesized
according to references 3, 4, and 5.
All CV and RDE voltammetry studies
were carried using PAR Model 263A Potentiostat – Galvanostat (PerkinElmer) with
three electrodes system consisting of Pt working, Pt wire counter, and Ag/(0.01
M) silver hexafluorophosphate (AgPF6)-CH3CN reference
electrodes. The Pt working electrodes for CV and RDE experiments were 3.0 mm
diameter (CH instrument Co.) and 1.1 cm (Pine Instrument Co.) disks, respectively.
The Pt working electrodes were polished with 0.05 mm alumina (Buehler), washed with deionized water, sonicated for
about 5 minutes, and dried before use. All experiments were carried out using
1.0 mM solutions of the complexes with 0.20 M tetrabutylammonium
hexafluorophosphate (TBAPF6) as the supporting electrolyte in
dimethylsulfoxide (DMSO). The solutions were bubbled with N2 prior
to the electrochemical measurements and blanketed with N2 while
conducting the experiments.
SEM study
was performed using an AMRAY 1810T microscope with tungsten filament running at
20 kV. The EDS system used was an Oxford Instruments Pentafet detector
with a SATW window for analysis down to boron. The energy range typically
examined was 1 to 15 kV with a resolution of 134 eV at 5 keV.
Shown in
<> 1. Davies, G.; El-Sayed, M. A.; El-Toukhy, A., Chem.
Soc. Rev., 1992, 101.
2. Caulton, K. G.; Davies, G.; Holt, E. M., Polyhedron,
1990, 9, 2319.
3. Dieck, H. T., Inorg. Chim. Acta, 1973, 7,
397.
4. El-Toukhy, A.; El-Essawi, M.; Tawfik, M.; El-Sayed, L.;
Iskander, M. F., Trans. Met. Chem., 1982, 7, 158.
5. El-Sayed, L.; El-Toukhy, A.;Iskander, M. F., Trans.
Met. Chem., 1979, 4, 300.
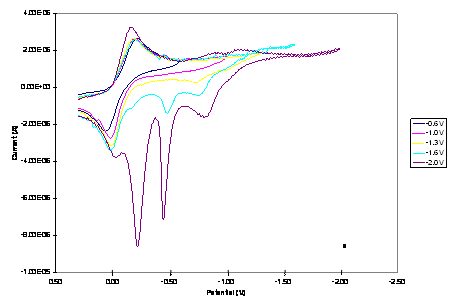
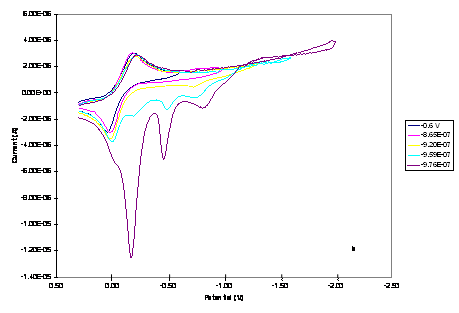
Figure 1. The effect
of switching potential on CV of 1.0 mM of the (a) Cu2Co2
and (b) CuCo3 core complexes in 0.20 M TBAPF6-DMSO at a
scan rate of 0.02 Vs-1.
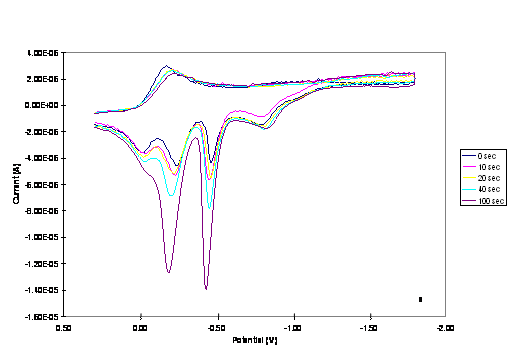
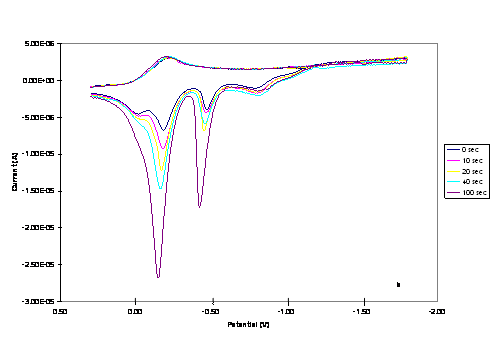
Figure 2. CVs of 1.0 mM of
(a) Cu2Co2 and (b) CuCo3 core complexes in
0.20 M TBAPF6-DMSO at a scan rate of 0.02 Vs-1.
Increasing peak currents correspond with pause time of 0, 10, 20, 40 and 100 s.
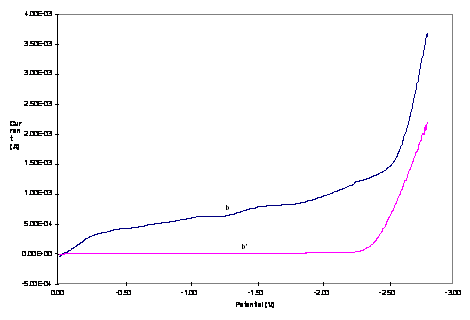
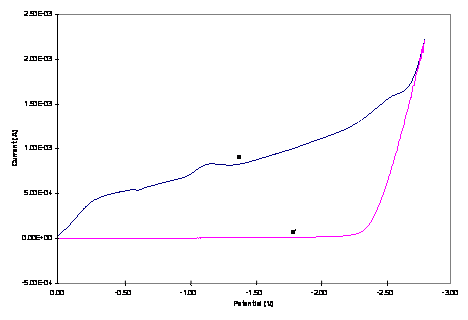
Figure 3. Hydrodynamic RDE
voltammograms of 1.0 mM (a) Cu3Co and (b) Cu2Co2
core complexes in 0.20 M TBAPF6-DMSO (a
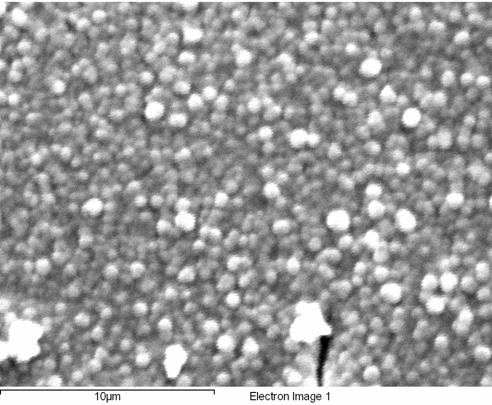
Figure 4. SEM micrograph of
the deposited film from 1.0 mM of Cu3Co core complex in 0.20 M TBAPF6-DMSO
at a potential of - 1.80 V and rotation rate of 1600 rpm.