46436-G10
The Oxidation of Ethanol and Dimethyl Ether by Binary and Ternary Electrocatalysts for Fuel Cell Applications
Introduction:
Proton exchange membrane fuel cell (PEMFC) hold great promise for applications such as portable power supplies and transportation. To unlock the promise of PEMFCs several challenges need to be addressed. These include sustainable fuel sources, fuel storage, cost and long term reliability.
The use of hydrogen as a fuel in the PEMFC has been documented to have safety, low energy density (0.8 kWh/kg at 3% wt% storage) and storage problems. There has been increasing interest in the past few years to develop direct alcohol/air PEMFC (DAFC) as an alternative to the pure hydrogen/air PEMFC to address the hydrogen fuel/hydrogen storage problem. Liquid fuels such as low-weight alcohols (in particular, methanol and ethanol), have advantages compared to pure hydrogen because they can be easily handled, stored and transported using the present gasoline infrastructure with only slight modifications. In addition, their energy densities are comparable to gasoline, varying from 6 KWh/kg for methanol to about 10KWh/kg for heavy alcohols.
Methanol is the most researched of the alternative fuels to hydrogen for the PEMFC; however, methanol has some drawbacks. Ethanol has little toxicity, is produced as a primary fuel, is renewable and has a theoretical energy density of about 8 KWh/kg. The use of ethanol in PEMFC addresses some of the problems encountered with methanol usage, however, the drawbacks of operating with alternative fuels, namely low activity of electrocatalysts, anode poisoning and fuel crossover has to be addressed.
In this project, new combinations of elements are being explored to increase the activity of catalysts for ethanol oxidation. Ternary and quaternary component electrocatalysts are being investigated.
Experimental Method:
Pt and Pt-based electrocatalysts were prepared by the modified Pechini-Adams method. Metal resins were prepared by first mixing citric acid (CA) with ethylene glycol (EG) at about 60oC. Appropriate amounts of metal salts (M) were dissolved isopropanol and then added slowly to the CA and EG mixture to obtain M: CA: EG molar ratio of 4:16:1. The CA, EG and metal salt solution mixture was then heated at about 90oC to half the initial volume. Appropriate volumes (based on the weight percent of metal expected in catalyst) of the different metal resins were measured into a crucible. Powdered carbon (Vulcan XC 72R) pretreated in nitrogen atmosphere was added to metal resin mixture to give a catalyst loading of 40 wt%. The mixture was sonicated for 10 minutes and then heated through three temperature regimes in a muffle furnace. Mixture was heated from room temperature to 250oC at1oC/min and held there for 1 hr. The temperature was then raised to 350oC at 15oC/min and kept there for 30 minutes; and finally to 450oC at 30oC/min and held there for 2 hrs. Upon cooling, electrocatalysts were again heated to 500oC for 11/2 hrs to rid them of excess carbon. Electrocatalysts were characterized by EDS, XPS and CVs (using 0.5M H2SO4 and 1M C2H5OH).
Results:
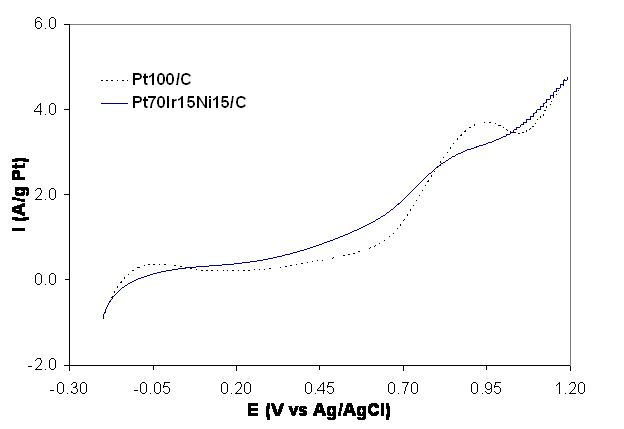
The oxidation
sweep of cyclic voltammograms in the presence of ethanol
for Pt/C and one tertiary catalyst PtIrNi with a
weight percentage of 70:15:15 are presented in Figure 1. It is readily apparent that the tertiary
catalyst increases the rate of ethanol oxidation at low potentials (0.2 vs. 0.8
V vs. Ag/AgCl).
The tertiary catalyst produced 88% more current per gram of platinum
than pure platinum at 0.7 V vs. Ag/AgCl. Figure 1: Cyclic voltammetric results of anode electrocatalysts
from ethanol oxidation at 25oC and scan rate of 100mV/s in 0.5M H2SO4
with 1M C2H5OH.
Other tertiary
and quaternary catalysts have been formulated and tested with mixed
results. To understand these results,
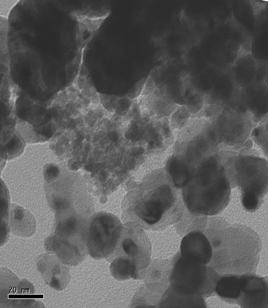
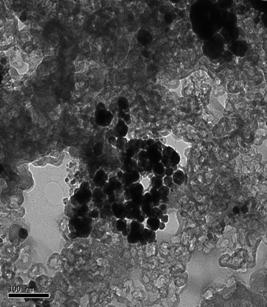
physical characterization of the catalysts has commenced. An example of the physical characterization
being performed is transmission electron microscopy with sample images shown in
Figure 2 for the Pt70Ir15Ni15 and Pt100.
From images like these, the distribution of particle sizes of the
catalysts is determined. X-ray
photoemission spectroscopy has been performed to analyze the surface
composition of several catalysts and the results are still be analyzed.
Pt70Ir15Ni15 Pt100 Figure 2. TEM Images of
Pt70Ir15Ni15/C and Pt100/C catalysts.
Summary: Research is continuing on the investigation
of ternary and quaternary catalysts to address the problems of low activity during
ethanol oxidation. Initial results are
promising as some tertiary catalysts show up to an 88% increase the rate of
ethanol oxidation at lower overpotentials.