47027-G5
Rational Self Assembly of Macromolecular Arrays for Optimized Light Harvesting and Photocatalytic Hydrogen Production
The research supported by this grant has made significant progress in the past year. To date, funds from this grant have been used only to pay the summer salary of the PI as stipends for the students conducting the research as well as supplies and materials were generously paid for by Bryn Mawr College. The research accomplishments to date include successful syntheses of both organic ligands and of transition metal complexes (TMCs), solution-based fluorescence quenching studies to examine electron and energy transfer, quantitative measurements of hydrogen produced from the photocatalytic reduction of water and electrochemical examinations of the thermodynamics and kinetics of adsorption of thiol-terminated TMCs on noble metal electrode surfaces.
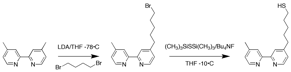
The synthetic scheme below, which required some modification from literature procedures, has been used to successfully synthesize the desired bipyridine ligand functionalized with a thiol-terminated alkyl chain.
Figure 1.
Synthetic scheme for the synthesis of thiol-terminated bipyridine ligand. To date, the
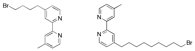
thiol-bipyridine ligand shown above, as well as the bipyridines shown below
functionalized with a bromine-terminated alkyl chain (which will serve as the
precursors to additional thiol-bipyridine ligands), have been synthesized.
Figure 2. Additional
thiol-bipyridine precursors that have been synthesized. These syntheses, which are
crucial for this work have been carried out by undergraduate researchers (Amy
Case '08, Erica Lo '09 and Suzanne Ali '09) who have, in the process of moving
this research forward, acquired a significant skill set in the realm of organic
synthesis. Using the TMCs (without the
thiol functional group) described in the original proposal, solution-phase
fluorescence quenching experiments were used to determine which combinations of
photosensitizers and electron relays lead to the most efficient light
harvesting and hydrogen production. This will allow the work on mixed
monolayers and on macromolecular assemblies to be targeted to the most
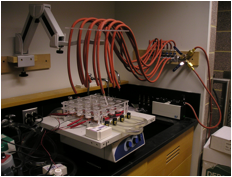
promising leads. We also designed and built the apparatus shown below at left for
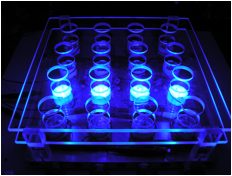
irradiating photocatalyst-containing solutions to produce hydrogen. The panel below
at right shows the apparatus with 4 positions activated.
Figure 3.
16-well photoreactor for the photocatalytic production of hydrogen. We carried out hydrogen
production experiments on the same combinations of photosensitizers and
electron relays as investigated by the fluorescence quenching experiments
described above. The photosensitizer/electron relay pairs that showed the
strongest fluorescence quenching behavior also led, as expected, to the
greatest amount of hydrogen produced. The full extent of this
project requires the synthesis of numerous TMCs with thiol-terminated
bipyridine ligands. As this project is still in its early stages, we are
synthesizing only a few of the necessary complexes in order to work out the
details of how to perform the necessary spectroscopic, electrochemical and
other experiments. We have successfully synthesized the ruthenium complex shown
below and while we do not yet have conclusive evidence, we believe that we have
also managed to make the cobalt complex shown below.
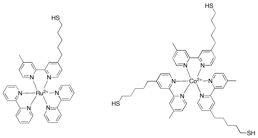
Figure 4.
Thiol-terminated ruthenium (II) bipyridine complex (left) and thiol-terminated cobalt
(II) bipyridine complex (right) synthesized to serve as photosensitizer and
electron relay, respectively, in surface-confined arrays for the photocatalytic
reduction of water to hydrogen. The goal of this project is
to design macromolecular arrays, using the self-assembly of thiol-terminated TMCs,
as catalysts for enhanced light harvesting efficiency. To that end, we have
begun experiments to fully understand the self-assembly process of these TMCs. Cyclic
voltammetry at a gold working electrode allows the adsorption of these
complexes on the electrode surface to be quantitatively monitored in real time,
giving access to information about the kinetics of adsorption. By varying the
concentration of the analyte, the thermodynamic driving force for the
self-assembly process can be measured. While we are still working on these
experiments for the cobalt complex shown above, we have completed them for the
ruthenium complex in Figure 4. These data indicate that the thiol functionality
allows the ruthenium complex to adsorb to gold with a free energy of adsorption
of approximately -48 kJ/mol and that the maximum surface coverage is
approximately 1.6 x 10-10 mol/cm2. The coverage versus
time profile for the adsorption process indicates that a kinetic barrier to
adsorption (as opposed to mass transport issues) is the limiting factor in
formation of functionalized surfaces. Kristin Kurek, a graduate student,
carried out the electrochemical, hydrogen production and fluorescence quenching
work described above. Work is ongoing to
synthesize additional thiol-terminated TMCs, to use electrochemical techniques
to characterize monolayers composed of multiple species of thiol-terminated
TMCs, to measure the rates of electron transfer between the electrode surface
and adsorbed TMCs and to design and implement the necessary apparatus for
spectroelectrochemical studies. The PI is extremely grateful to have been
awarded this grant as these funds have been crucial in moving this project
forward, allowing the PI to be able to train and mentor the diverse group of
students involved in this work.