

42402-B5
Using Model Systems to Enhance Understanding of Silica Surface Reactions
Aminosilane-modified silicas are widely used in various technologies. The homogeneous reaction of 3-aminopropyldimethylmethoxysilane (APDMS) with a silsesquioxane silanol in the solvents hexane and THF were studied to model the same organosilane reaction with silica.
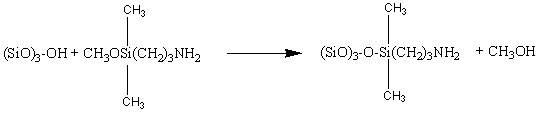
This reaction exhibits interesting solvent dependent and temperature dependent kinetics behavior.
Addressing the solvent dependent behavior first, the reaction occurs much faster in hexane than THF solution. (The same trends are seen for reactions with silica in the same solvent slurries.) Computational studies of this reaction using Gaussian and GAMESS show that the reasons for this are twofold: 1) THF exhibits a stronger competitive effect on the formation of the APDMS/silsesquioxane pre-reaction complex; and 2) H+ transfer (the reaction rate limiting step) in the autocatalytic reaction is less favored in THF because of the poorer electron-donor properties of THF than APDMS.
The initially puzzling temperature dependent kinetics behavior is largely a consequence of the pre-reaction complex formation. The figure below shows second order fits to the data (obtained by FTIR) as a function of temperature.
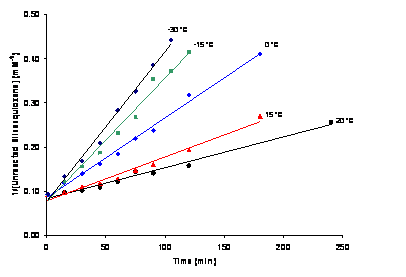
The slope of these lines (i.e. the rate constant) increases with decreasing temperature. This is explained by the decrease in APDMS/silsesquioxane pre-reaction complex formation with increasing temperature. As temperature is further decreased below -30 °C, the reaction rate decreases as would be predicted from a reaction rate limited reaction. Thus at higher temperatures the reaction is pre-reaction complex formation limited, and at lower temperatures it is H+ transfer (i.e. reaction) rate limited. These results suggest that the reaction rates of aminosilanes with silica can be increased by decreasing temperature; a potentially important result for the industrial scale synthesis of these materials.
Analysis of low temperature experimental data and computational results suggests that the reaction can be of both first and second order with respect to APDMS. The two reaction mechanisms corresponding to these results are shown below.
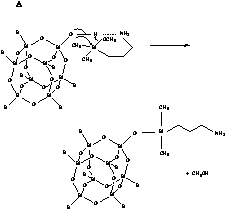
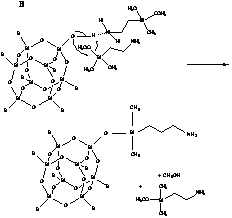
In the former, coil conformation (A) of the Si(CH2)3NH2 chain allows the N atom from the same APDMS molecule to promote the H+ transfer. In the case of a linear conformation of this chain (B), the second APDMS molecule plays the role of catalyst. Both APDMS conformations differ insignificantly in the total energy and Gibbs free energy of solvation. This complexity of the APDMS/silsesquioxane reaction results in a broad distribution of the activation energy, which is found to be around 45 kJ/mol.
Two students were provided the opportunity to work over the summer in 2008 on this and a related project from PRF funds. This work has had a strong and positive impact on a hard working undergraduate. This student has become very excited about research, having never considered it possible that he would be coauthoring publications. Directly as a result of this experience, he has changed career paths and decided to enter graduate school rather than the work force as a B.S. chemist. It should be noted that two undergraduates who have previously been supported on this grant are now enrolled in strong Ph.D. programs in chemistry at U. Wisconsin-Madison, and U. Illinois Urbana-Champaign. The other student supported on this grant in 2008 was a very capable M.S. student, who will enroll in a Ph.D. in chemistry program upon graduation. PRF funding has been a huge help to my career; it has not only increased my ability to do science and get published, but also has made it possible to positively impact the lives of undergraduate (and now M.S.) students. For this I am very grateful.