45787-G6
The Ignition of the Butanol Isomers
Summary
A
new shock tube has been designed, constructed, characterized, and validated for
high-temperature combustion chemistry experiments. The first experiments
performed in the new shock tube were a comprehensive study of the ignition of
the four butanol isomers, possible future high-octane rated additives for
gasoline. The difference in reactivity of the four butanol isomers was
experimentally characterized and in conjunction with kinetic modeling efforts
of a collaborator (F. Battin-Leclerc and co-workers,
New Shock Tube A
new shock tube has been designed and constructed for the high-temperature
investigation of combustion chemistry. The fully instrumented stainless steel
shock tube has a 12.3 cm inner diameter, a 7.5 m long driven section, a 3.1 m
long driver, and is capable of test pressures of 0.1-10 atm; see Figure 1 for
images of the shock tube. The shock tube has been fully characterized and
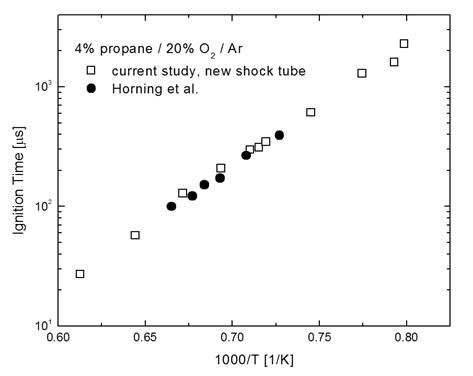
validated. Validation was performed by measuring ignition delay times for
propane/oxygen/argon mixtures and comparing to the measurements of Horning et
al. (2002), which we have a high level of confidence in; see Figure 2 for a
comparison.
Figure 1.
A photo of the new RPI shock tube.
Figure 2. Comparison of the previous Horning et al. (2002)
propane shock tube ignition measurements to validation measurements made in the
new RPI shock tube facility.
Butanol Ignition Butanol
has received recent interest as a possible future high-octane rated additive
for gasoline. Therefore, the autoignition of the
four isomers of butanol (1-butanol, 2-butanol, iso-butanol,
and tert-butanol) has been experimentally studied at
high temperatures in a shock tube. Ignition delay times for
butanol/oxygen/argon mixtures have been measured behind reflected shock waves using
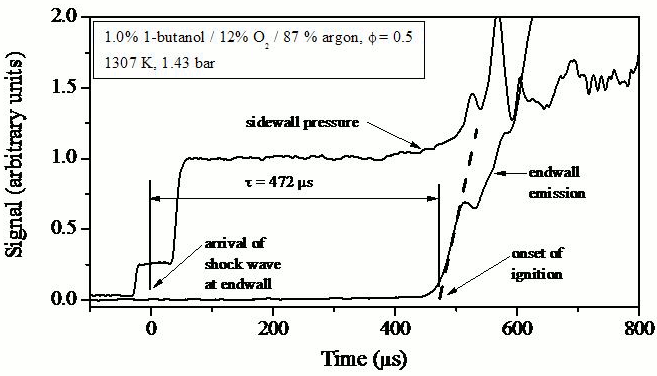
electronically excited OH emission and pressure measurements to determine
ignition delay times; see Figure 3 for an example measurement and Figure 4 for
example ignition time results. A detailed kinetic mechanism has been developed
by our collaborators at
Figure 3.
Example butanol ignition delay time measurement (pressure and
OH* emission).
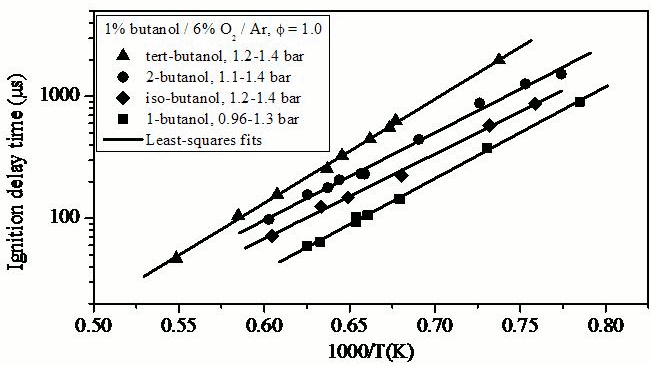
Figure 4.
Ignition time measurements for all four butanol isomers for a mixture
composition of 1% butanol / 6% O2 / Ar
(Ф = 1.0) and reflected shock pressures near 1 bar.
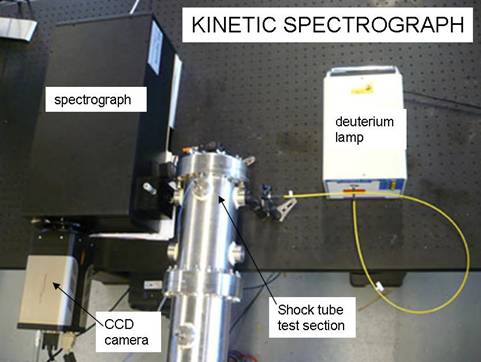
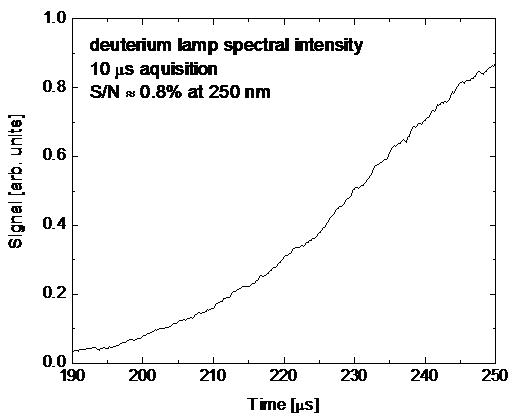
Kinetic Spectrograph An
ultraviolet kinetic spectrograph has been purchased, assembled, and initially
tested for the future measurement of ultraviolet absorption spectra (200-400
nm) in shock-heated gases at high speed (100 kHz and faster). The kinetic
spectrograph consists of a high-powered fiber-coupled deuterium light source
that is transmitted through shock-heated gases of interest; the light is
dispersed with a 500 mm spectrograph, and recorded on a CCD camera. In order to
record multiple spectra at a high repetition rate, only the top few rows of the
CCD array are exposed to the dispersed light. The CCD array is binned in a
manner that allows the exposed CCD rows to be recorded and quickly transferred
to the unexposed portion of the array. The process is repeated until the entire
CCD array is full of spectra, each of which is separated in time by 2-10 μs. A photograph of the kinetic spectrograph is shown
in Figure 5 and an example spectral intensity measurement is shown in Figure 6.
This diagnostic tool will be used, in the next few months, for the originally
proposed study of the ultraviolet absorption and oxidation kinetics of phenyl
radicals in shock-heated gases. Phenyl radical oxidation, C6H5
+ O2
Figure 5.
A photo of the kinetic spectrograph.
Figure 6.
Example spectral intensity measured with the kinetic spectrograph.