43746-G2
Controls on the Hydrogen Isotopic Composition of Petroleum Hydrocarbons
The goal of this project has been to understand the process of hydrogen isotopic exchange in organic molecules from sedimentary environments. Such processes affect even C-bound H over million-year timescales at temperatures above ~80°C, and thus potentially impact the D/H ratios of petroleum hydrocarbons. Because this exchange is so slow, laboratory experiments on simple hydrocarbons are not feasible even at elevated temperatures. Our approach has been to combine laboratory incubation experiments of labile molecules with ab initio density functional theory (DFT) calculations for more recalcitrant hydrocarbons. We have conducted exchange experiments on simple ketones, taking advantage of rapid keto-enol tautomerism under base catalysis to achieve equilibration of the adjacent (a) hydrogens over several days. The results of these experiments then provide a calibration dataset for DFT estimates of vibrational frequencies, which can be used to directly calculate equilibrium D/H fractionations for hydrocarbon moieties of choice.
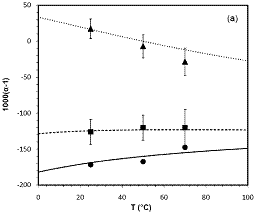
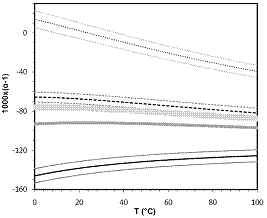
Experimental incubations were conducted for 7 ketone substrates, included both linear and cyclic compounds at temperatures from 25 to 70°C. The experimental data yield equilibrium fractionation factors ranging from 0.802 (cyclohexanone at 70°C) to 1.027 (2,4-dimethyl-3-pentanone at 25°C), with fractionations increasing in the order: cyclic 2° < linear 1° < linear 2° < linear 3° (Figure 1). Experimental and DFT fractionations are in excellent agreement for these compounds, with a correlation coefficient (R2) of 0.979. Estimates of fractionations for hydrocarbon H based on DFT calculations are shown in Figure 2. In general, we predict that n-alkanes will be depleted in D relative to water at equilibrium by 80 to 100‰, regardless of temperature. The very weak temperature dependence was unexpected, and derives from the opposite temperature dependence of effects at 1° versus 3° positions. This dataset now provides a quantitative basis for interpreting organic D/H ratios in mature sediments, oils, and gases. More specifically, it will be possible to assess whether differing organic structures are in isotopic equilibrium and thus have been subject to extensive hydrogen exchange, or whether they potentially preserve original environmental signals. A manuscript describing these results is currently in preparation.
One additional outcome of this project has been a more complete understanding of processes affecting accuracy and precision in GC-pyrolyis-IRMS. Because our experiments require very accurate measurement of organic D/H over a large range, we synthesized multiple organic standards having dD values ranging from -200 to +800‰. Careful manipulation of the order and D/H ratio of these standards allows us to show that there is a small, systematic memory effect in GC-pyr-IRMS systems, such that 2-4% of the hydrogen in each chromatographic peak is derived from that in the preceding peak. In experiments such as ours, where the desired results are obtained by varying D/H ratios over hundreds of permil, this memory can introduce a systematic bias of 20-40‰ in fractionation factors unless properly accounted for. A manuscript describing these effects is currently in review by Analytical Chemistry.
| |||
| |||