43124-B3
Magnetic Studies and Room Temperature Carbon-Carbon Bond Cleavage in Ruthenium Carboxylate Clusters
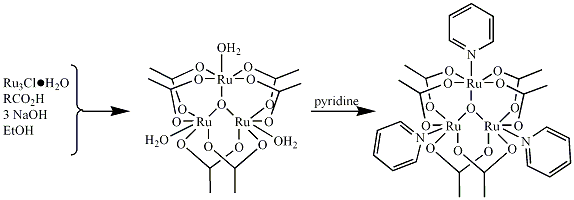
Our determination of a general strategy for the synthesis
and purification of oxo-bridged basic triruthenium carboxylate clusters
has resulted in the preparation and characterization of two butyrate species,
Figure 1. General strategy for the synthesis of basic
ruthenium carboxylates Our efforts to optimize the synthesis have correlated with
the report by Toma and coworkers,3
that this apparently simple system is actually extremely sensitive to pH, even
when carried out in organic solvents. By
using a generous amount of acid in the first step and by minimizing the
pyridine in the second step, the desired blue complexes may be produced
reliably. Our experiments have
determined that in the presence of less acid in the first step and more base in
the second, that a green product, hypothesized to be the neutral complex, The extensive redox
behavior of the {Ru3O(O2CR)6}n+
clusters has long been a source of the interest in these systems since the
extensive electron delocalization results in the stability of multiple
different charges for the cluster. With
our isolation and characterization of two new carboxylate
clusters in this series, the effect of the change in carboxylate
ligand from the acetate to a longer chain may be
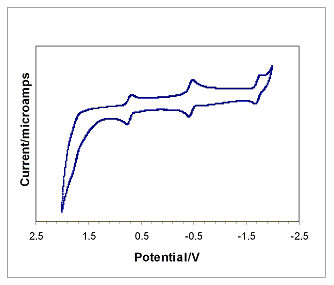
observed. Both the isobutyrate
and n-butyrate clusters display three widely spaced reversible one-electron
couples and a fourth poorly resolved process as seen in Figure 2. Compared to the acetate data collected under
similar conditions, the potentials of the reductions from an overall cluster
charge of +1 -> 0 (-0.23 V) and 0 -> -1 (-1.50 V) are noticeably more negative,
corresponding to the increased pKa of the butyric acids. In contrast, the reduction potential for the
+2 -> +1 cluster charges is consistently around 0.95 V for all of the
clusters, indicating that the pKa effect is negligible for electron transfer
between the higher oxidation states.
Figure 2. Cyclic voltammetry
for This grant award has had substantial impact on my students
and References 1. C. C. Pink,* N. L.
Saad,* J. M. Schlough,* J.
L. Eglin, and L. E. Pence, submitted to 2. C. C. Pink,* N. L.
Saad,* Mugge, A. M.*; Svenson, A.*; Pence, L. E., manuscript in preparation.
3. Nunes, G. S.; Alexiou, A. D. P.;
Araki, K.; Formiga, A. L. B.; Rocha, R. C.; Toma, H. E. Eur. J. Inorg. Chem. 2006,
1487-1495.