45215-AC10
Synthesis and Polymorphic Control for Visible Light Active TiO2 Nanoparticles
Burtrand I. Lee, Clemson University
Synthesis and Polymorphic Control for Visible Light Active TiO2 Nanoparticles
1.0
During the 2nd year, our research
focused on improving the properties and visible light active (VLA) photocatalytic
activities (PCA) of as-synthesized titania by a solvent treatment called solvent-based
ambient condition sol (SACS).
The nomenclature used, preparation conditions, and % phase
composition of titania samples are shown in Table 1 for N-methylpyrrolidone
(NMP) treated titanias.
Table
1 Experimental Conditions 1
Sample ID % Content of TiO2 Phase (wt%)a Mode of Formation Preparation or Post-treated Temperature (°C) Preparation or Post-treated Time (hr) A B R WACS 48 50 2 WACS 83 15 WACS-200 45 53 2 Calcination of WACS 200 2 SACS 42 55 3 bSACS of WACS 170 4 SACS-200 44 53 3 Calcination of SACS 200 2 P25 79 - 21 High T flame oxidation N/A N/A a Obtained from
XRD data
b U.S. Patent applied & info withheld.
1.1.2 Characterizations All samples were characterized by XRD, N2 physisorption,
CHNS analysis, UV-Vis spectrophotometry, and FT-IR. The
PCAs were evaluated by the degradation of the methyl orange (MO) under visible
light (VL) irradiation 1-4.
1.2 Table 2 summarizes the crystallite sizes, the average
particle size, and the surface area analysis results of titania samples. The
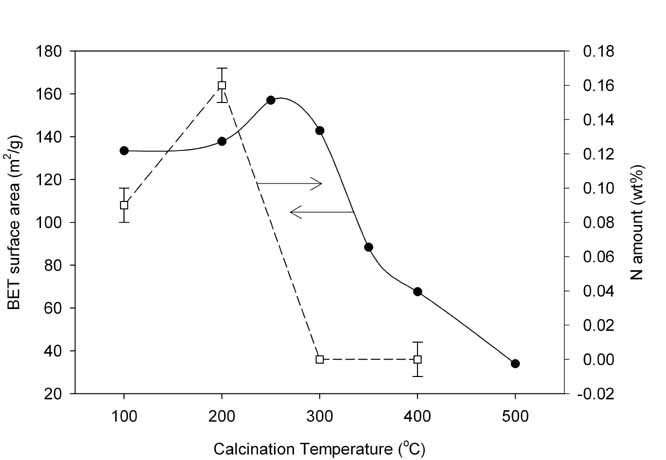
effect of the calcination temperature of SACS samples on the surface area and
the nitrogen content in the samples are given in Figure 1. All NMP and nitrogen
in SACS samples were released by the calcination above 250oC which is
supported by CHNS analysis.
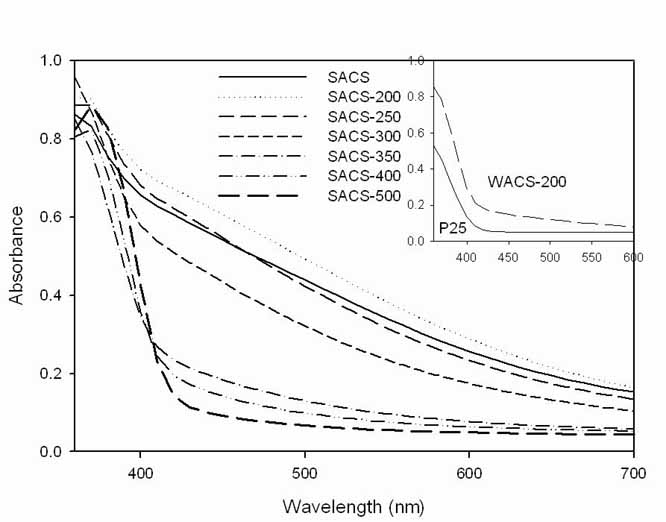
SACS
samples exhibited VLA potential by the shifts of the absorption shoulders to
the VL region, compared with P25 and WACS-200 in the UV/Vis spectra in Figure 2.
The more nitrogen present in the samples, given by CHNS analysis, the greater the
VL absorption by narrowing of titania band gap 5. SACS treatment is also a useful method to extract lattice hydroxyls which showed an adverse effect on the PCA 1,3.
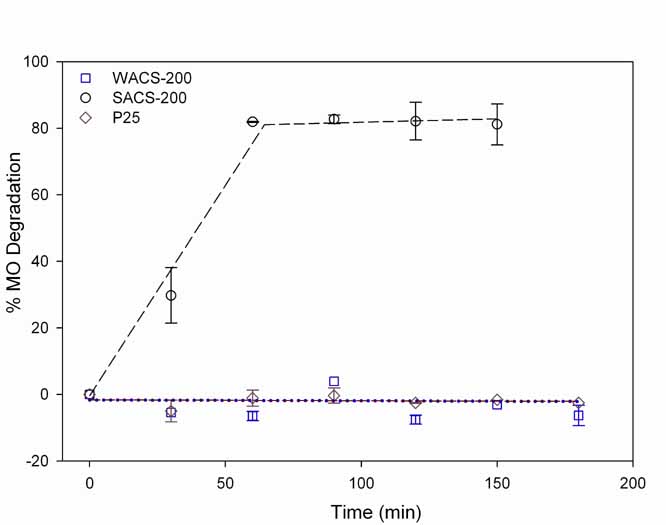
Figure 3 depicts the PCA of titania samples compared to P25.
Nitrogen doping in SACS-200 showed a significant band gap narrowing (see UV/Vis
spectra) which resulted in an increase in VL-PCA. SACS-200 has a much higher VL
PCA than WACS-200 and P25. WACS-200 and P25 with no nitrogen are not VLA
catalysts.
Table 2 The physical properties
Sample ID Crystallite size (nm)a Average particle size (nm)b BET surface area (m²/g)c Anatase Brookite Rutile WACS 6 8 19 6 163 WACS-200 6 7 18 7 157 SACS 7 8 12 6 133 SACS-200 6 8 11 7 138 P25 21 - 40 40 56 a Calculated from XRD data using the Scherrer equation.
Error of measurement = ±5%.
b Determined by using TEM micrograph.
c Using N2
physisorption at 77K. Error of measurement = ±10%.
Figure
1 Effect of calcination for SACS samples on surface area and nitrogen
content.
Figure 2 UV/Vis spectra of as-prepared titania
samples and P25.
Figure
3 MO degradation under VL irradiation
2.0 We found that the SACS-NMP is an effective method to extract
lattice hydroxyls and nitrogen to be incorporated in the crystal lattice.
SACS-200 showed the best PCA under VL irradiation among the prepared titania
samples and P25. Although the NMP treatment is not a doping in the conventional
sense nor the NMP is a photosensitizer, it is shown to be effective for VLA
photocatalyst. The stability of NMP treated titania in aqueous environment is being
investigated.
2.1 Personnel Impacts The PI and his coworkers were able to produce three peer refereed journal papers in addition to a U.S. patent application. We feel this is an excellent performance for the PI, graduate students and undergraduate students. In this project six undergraduate students synergistically worked together. For them it was life time research experience. To
graduate student, the PRF financial support was an essential part of the
graduate student's life in pursuing her Ph.D. She has gained much more experience
in this emerging and hot research field “Visible light active TiO2”.
The research experience and accomplishments will help her to get a satisfactory
job easily upon graduation
3.0 References
(1) Kaewgun, S.; Nolph, C. A.; Lee, B. I. Catalysis Letters 2008, 123, 173.
(2) Kaewgun, S.; Mckinney, D.; White, J.; Smith, A.; Tinker, M.; Ziska, J.; Lee, B. I. J. Photochemistry and Photobiology A: Chemistry 2008.
(3) Kaewgun, S.; Nolph, C. A.; Lee, B. I.; Wang, L.-Q. Materials Chemistry and Physics 2008.
(4) Nolph, C. A.; Sievers, D. E.; Kaewgun, S.; Kucera, C. J.; McKinney, D. H.; Rientjes, J. P.; White, J. L.; Bhave, R.; Lee, B. I. Catalysis Letters 2007, 117, 102.
(5) Aita, Y.; Komatsu, M.; Yin, S.; Sato, T. J. Solid State Chemistry 2004, 177, 3235.