

44762-B1
Asymmetric Catalytic Hydroamination of Aminoallenes and Aminoalkenes by Chiral Titanium Amide-Alkoxide Complexes
We have been studying the intramolecular hydroamination of substituted aminoallenes with chiral titanium amino-alcohol derived catalysts. The ligands are prepared in two steps from natural or unnatural amino acids and are enantiomerically pure. We performed the hydroamination reaction catalytically with an enhanced regioselectivity for a-vinylpyrrolidine products over other titanium catalysts, with enantiomeric excesses of up to 16% for our first generation ligands (R' = H). The aims over the last year were to improve upon our initial results through systematic ligand tuning.
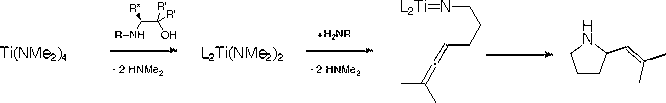
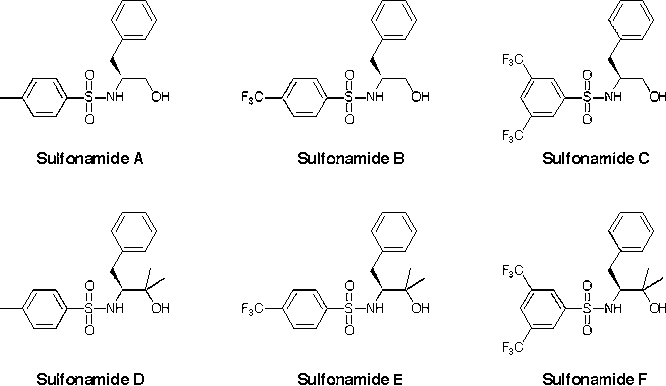
Our first goal was to prepare ligands with increased steric bulk (R' = Me, Bu or Ph) by alkylating with the appropriate Grignard reagent instead of reduction with LiAlH4 during the ligand synthesis. Except for one derivative that has resisted multiple attempts at synthesis (R = iPr, R* = CHMe2, R' = Ph), we have completed the series and have synthesized and characterized the remaining 17 ligands.
The substrates for the hydroamination reaction must be synthesized in a six-step procedure, and we sought a more rapid screen for catalytic activity by investigating the alkylation of benzaldehyde with diethylzinc. Our ligands and their titanium complexes catalyze this reaction, and since the substrate is commercially available, it is a more rapid screen for enantioselectivity. When this reaction is carried out with our first generation ligands (R' = H), we observe enantiomeric excesses below 10% in most cases. However, increasing the steric bulk (R' = Bu or Ph) generally increases the ee values significantly, with values near 75% for our best ligands.
We then continued our investigation of the catalytic intramolecular hydroamination with an aminoallene substrate. We used 6-methyl-hepta-4,5-dienylamine as our substrate (as shown in the figure) as it gives only a single product. Enantioselectivities were measured for the 17 newly prepared ligands; however, there is not a substantial increase in enantioselectivity with the second generation ligands in this reaction as was seen for the benzaldehyde screen. This led us to two conclusions. First, that the benzaldehyde reaction is not a good catalytic screen for the hydroamination. The second is that we require more substantial ligand tuning to achieve higher enantioselectivity in this reaction.
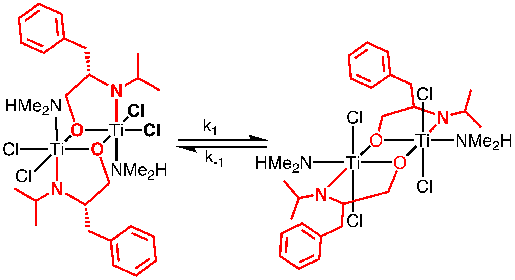
We have also continued to examine the coordination chemistry of the amino-alcohol ligands (H2L) with TiCl2(NMe2)2. The complex that is formed has resisted crystalization and we do not know its molecular structure. However, NMR and combustion analysis suggest its formulation as [TiCl2(L)(HNMe2)]2. The complex also exhibits fluxional behavior by NMR spectroscopy. Preparation of the deuterated derivative by starting with d6-acetone in the ligand synthesis simplified the NMR spectrum of the resulting complex. Modeling of the fluxional NMR behavior through full lineshape analysis resulted in activation paramaters for the fluxional process of ΔH‡ of 16-20 kcal/mol, and ΔS‡ of 2-16 eu.