44953-G5
Direct Measurement of Casimir Force in Critical Films of Binary Liquid Mixtures
We performed
preliminary experiments in measuring the critical Casimir force of a binary
liquid mixture. We are using a home-built atomic force microscope (AFM) for
this purpose. Previously we used a single component liquid, a nearly spherical,
nonpolar molecule tetrakis(2-ethylhexoxy)silane (TEHOS) as a test for the
set-up. Our results indicated oscillations in the stiffness with a period of ~
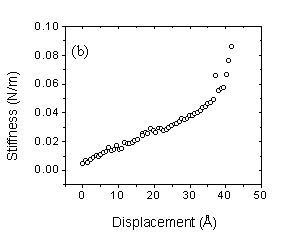
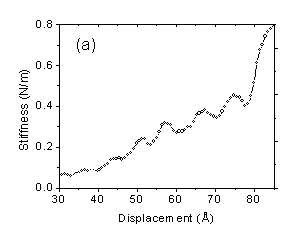
1 nm, which is consistent with the molecular dimension of TEHOS (Fig. 1a). For
the measurement of the Casimir force, we are using a mixture of water and 2, 6
lutidine, which constitutes similar (+,+) boundary condition. This system has a
convenient critical temperature, Tc Fig.
1: Stiffness of (a) TEHOS and (b) water + 2, 6 lutidine film as a function of
displacement. The surface is located to the right.
In
parallel, the support from the PRF grant had helped us to pursue other
experiments in parallel. We have used the method of fluorescence correlation
spectroscopy (FCS) to measure the center-of-mass diffusion coefficient of
fluorescently labeled polystyrene (MW=8100 g/mol) dissolved in
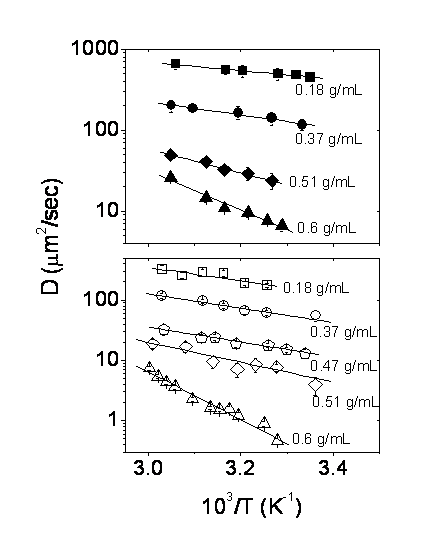
polymer/toluene solutions above the overlap concentration (Fig. 2). The
diffusion coefficient was found to decrease by
two orders of magnitude for a concentration change of 0.17 g/cm3 to
0.6 g/cm3 at 34 °C. Less dramatic changes were observed at higher
temperatures. The results are compared with the diffusion of free dyes in
similar concentrated solutions. Vrentas-Duda free volume theory can explain the
data reasonably well, from which the unit size of transport for the labeled
macromolecule and the dye relative to the solvent is determined. The activation
energy of diffusion was found to increase significantly as a function of
concentration.
Figure 2. Temperature
dependence of the diffusion coefficient for the free dye coumarin (top) and fluorescein-labeled PS (bottom), dissolved in varying
concentrations of PS in toluene. In the temperature regime investigated, the
data obey the Arrhenius equation (straight line fits).