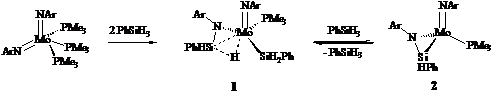Back to Table of Contents
45990-AC3
New Silanimine and NSi-H-M Agostic Complexes for Coupling with Petroleum Derived Products
Georgii I. Nikonov, Brock University
The final goal of this project is to develop new
synthetic methodologies for the coupling of silanes
with petroleum derived products. Specifically, it is aimed at the preparation
of new silanimine and agostic
silylamido complexes and investigation of their
reactivity towards unsaturated organic substrates.The specific results of the current grant period
are:1) For the first time we
accomplished the coupling of two silane molecules
with an imido complex. The product, (ArN=)(ArN-SiHPh-H...)Mo(SiH2Ph)(PMe3) (1), features both the agostic silylamido group and silyl functionality. Complex 1 was characterized by NMR and IR spectroscopy and by X-ray
diffraction.2) By
labelling experiments with PhSiD3 and TolSiH3, we showed
that complex 1 reversibly eliminates
silane PhSiH3 to generate a reactive silanimine intermediate (ArN=)(ArN-SiHPh)Mo(PMe3) (2) (Scheme 1). EXSY NMR confirms this
by showing that the silyl ligand
is an exchange with external silane.
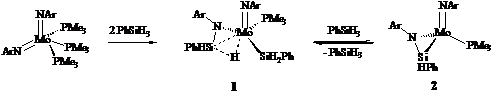
Scheme
1
3) We
discovered that 1 is an effective
catalyst for hydrosilylation of aldehyde
and ketones and alcoholysis
of silane. This is the first example of catalytic behavior
of an agostic silyl
compound. Moreover, we achieved catalytic hydrosilylation
of nitriles which is selective in the first step, the products being silylated
imines. Hydrosilylation of nitriles
is rarely done catalytically and in no previous case selectively. Complex 1 also catalyzes slowly silane redistribution when excess PhSiH3 is
present.4) We
performed stoichiometric reactions of 1 with olefins and nitriles
and in both cases observed products of Si-C coupling (Scheme 2). The reaction
goes via silane elimination to give intermediate 2, which then adds olefin to the silanimine ligand to give a five-
membered metalacycle.
Beta-hydride abstraction affords a silylated
olefin/hydride complex which was isolated in the case of substituted olefins,
such as styrene. With ethylene two equivalents are added to give an ethyl
complex, which can be converted to the hydride (ArN=)(ArN-SiHPh-CH=CH2)Mo(H)(PMe3)
by the reaction with PhSiH3. The latter product easily reacts with
olefins RCH=CH2 to give alkyl derivatives (ArN=)(ArN-SiHPh-CH=CH2)Mo(CH2CH2R)(PMe3).

Scheme
2
5) By
reacting the complex (tBuN)2Mo(PMe3)2 with H3SiPh
we achieved for the first time the
coupling of three silane molecules with an imido complex. The product, (tBuN=){h2- tBuN(SiHPh)2}Mo(H)(SiH2Ph)(PMe3)2
(3) was characterized by NMR
spectroscopy and X-ray diffraction. Note that 3 is formally a silyl hydride derivative
of Mo(VI) compound, i.e. has a very unusual oxidation state.This result proves conclusively
that an imido ligand can be
transferred from the metal to silicon, which may be a step in our proposed tricomponent coupling reaction of silane,
amine, and an unsaturated substrate. Thus, so far we observed three individual
steps of the proposed catalytic cycle: silane/imido coupling, coupling of the agostic
silylamide with unsaturated substrates (proceeding
via a silanimine), detachment of the functionalized imido ligand from the metal. The
remaining goal is to assemble these steps into a cycle on one specific metal
center.6) We
prepared new imido hydride precursors, (RN=)MoCl(H)(PMe3)3 (R=Ar
and Ar'), which were then used to synthesize tripod-supported
derivatives (PhL3)(Ar'N=)Mo(H)(PMe3)
(4, PhL3 = PhB(N-metylimidozolydene)), (Tp*)(Ar'N=)Mo(H)(PMe3)
(5, Tp* = tris(3,5-dimethylpyrazolyl)borate), and (Tp)(ArN=)Mo(H)(PMe3) (6, Tp = tris(pyrazolyl)borate). For
comparison purposes, we also prepared their Cp analogs Cp(RN=)Mo(H)(PMe3)
(R = Ar' (7)
and Ar (8)).7) We
studied reactions of the new tripodal complexes with silanes. Complexes 4
and 5 either do
not react with silanes or give intractable mixtures
of products (at elevated) temperatures, most likely due to the bulkiness of the
tripodal ligands. However,
complex 5 catalyses hydrosilylation of cyclohexanone
by H3SiPh at 70 șC. In contrast, less sterically
congested complex 8 reacts with one
and two equivs of H3SiPh to give the silyl complexes Cp(ArN=)Mo(SiH2Ph)(PMe3)
and Cp(ArN=)Mo(H)(SiH2Ph)2,
respectively. The unexpectedly high
oxidation state VI of molybdenum is observed in the latter product.
Back to top