
Back to Table of Contents
44412-GB1
New Synthetic Methods Using Oxime Ethers
Debra D. Dolliver, Southeastern Louisiana University
This PRF grant has
afforded the PI the opportunity to establish an active undergraduate research
program which has resulted in numerous presentations by students (five at
national ACS meetings, three at regional ACS meetings, and one at a Louisiana
Academy of Sciences meeting with an additional two scheduled for the Southwest
Regional meeting in October, 2008) and by the PI (three at national ACS
meetings). This work has resulted in one
publication in the Canadian Journal of Chemistry1 on nucleophilic substitution of N-alkoxyimidoyl fluorides by carbon nucleophiles which was primiarly
performed by undergraduate researchers.
In addition, one manuscript is currently being completed for publication
on work performed by undergraduates involving the synthesis of O-alkylazidoximes
and an investigation of their reactions in electrophilic
media.Under the term of this grant the research
group has investigated using N-alkoxyimidoyl halides as starting materials in the
synthesis of oxime ethers of pure E
or Z isomeric configuration, even in cases where there is little difference in
the thermodynamic stability of the two geometric isomers.1 This type of control of the geometric
configuration is of importance as oxime ethers in a
single isomeric form are useful starting materials for the formation of chiral amines.2-6Work has been published by the PI and her
undergraduate researchers showing that a single geometric configuration of an oxime ether is formed from nucleophilic substitution of a (Z)-N-alkoxyimidoyl
fluoride by a Grignard reagent in moderate to good yields (Scheme 1).
Scheme 1: Nucleophilic Substitution of a (Z)-N-alkoxyimidoyl fluoride by a Grignard Reagent
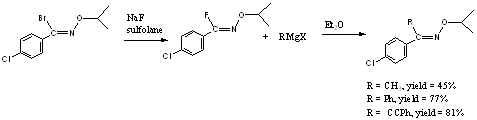
Additionally,
nucleophilic substitution of N-alkoxyimidoyl fluorides by enolate-type ions was also included in the previously-mentioned
paper. These substitutions result in
novel carbon acids which display varying degrees of imine-enamine
tautomerism (Scheme 2).
Scheme 2: Nucleophilic substitution reactions of
N-alkoxyimidoyl fluorides by enolate-type ions.
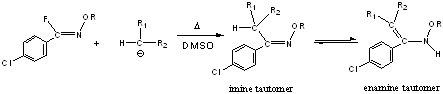
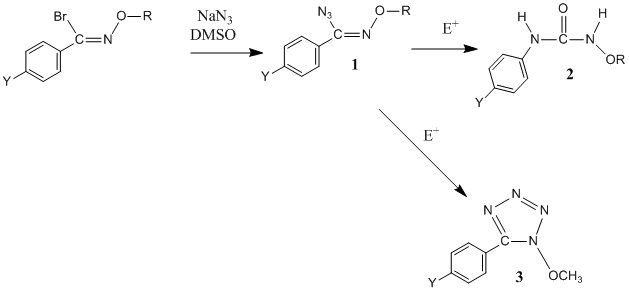
Work has been completed on the
synthesis of O-alkylazidoximes
(1, Scheme 3). X-ray crystallography on 1e revealed it to be only in the Z
configuration,7 so it is assumed that the
others are also in this configuration. Reaction
of 1 with an electrophile
results in two different products produced by two different pathways: a Schmidt-type rearrangement pathway to form a N-alkoxyurea (2) and
an isomerization-cyclization pathway to form an N-alkoxytetrazole
(3).
These two pathways have been
recognized for related compounds,8,9 but no
work has been performed to better understand the factors that influence the
path chosen. Our group has now completed
studies on the impact of the solvent and the electrophile
on the pathway preference. Our results
indicate that the type of electrophile is responsible
for the reaction pathway chosen. To
better understand the energetic parameters of both pathways, the PI has
successfully collaborated with a computational chemist (Dr. Thomas Sommerfeld) who has mapped out the features of both
mechanistic pathways, and a manuscript is being currently prepared for
publication on the work.
Scheme 3: Synthesis of an azidoxime
ether and competing reaction pathways in electrophilic
media.
Compound | R | Y | Isolated Yield |
1a | CH3 | H | 70% |
1b | CH3 | CH3 | 75% |
1c | CH3 | OCH3 | 42% |
1d | CH3 | Cl | 61% |
1e | CH3 | NO2 | 87% |
1f | i-Pr | Cl | 85% |
This grant's support has allowed the
PI and her students to interact with the greater chemistry community, to learn
about advances in related research, and to establish
contacts with researchers and collaborators at other universities. The students also have learned about graduate
school and career opportunities in chemistry.
References:
- Dolliver, D. D.; Delatte, D. B.; Linder, D. B.; Johnson, J. E.; Canseco, D. C.; Rowe, J. E. Can. J. Chem. 2007, 85, 913-922.
- Moody, C. J.; Gallagher, P. T.; Lightfoot, A. P.; Slawin, A. M. Z. J. Org. Chem. 1999, 64, 4419-4425.
- Gallagher, P. T.; Hunt, J. C.A.; Lightfoot, A. P.; Moody, C. J. J. Chem. Soc., Perkin Trans. 1 1997, 2633-2637.
- Bloch, R. Chemical Reviews 1998, 98, 1407-1438.
- Dieter, R. K.; Datar, R. Can. J. Chem. 1993, 71, 814-823.
- Enders, D.; Reinhold, U. Tetrahedron: Asymmetry 1997, 8, 1895-1946.
- Dolliver, D. D.; Smith, S.; Delatte, D. B.; Patel, K. D.; Thomas, T. E.; Chagnard, J.; Johnson, J. E.; Canseco, D. C.; Fronczek, F. R.; Bryan, C. D; Muller, J. R.; Rowe, J. E.; McKim, A. J. Chem. Crystallogr. 2007, 37, 837-846.
- Hegarty, A. F.; Aylward, J. B.; Scott, F. L. Tetrahedron Lett. 1967, 14, 1259-1262.
- Plenkiewicz, J. Tetrahedron Lett. 1975, 5, 341-342.
Back to top






