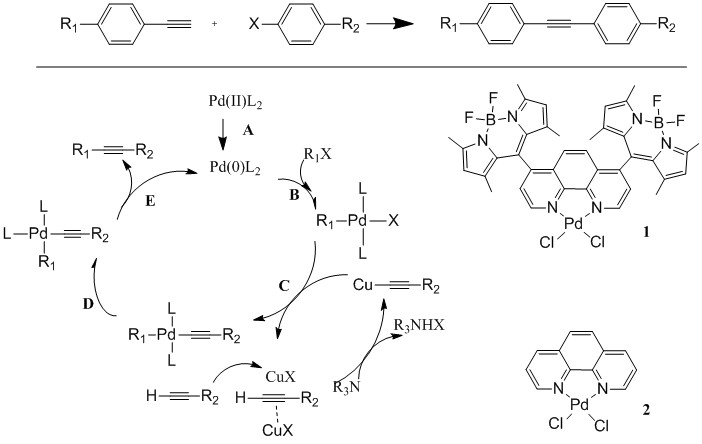
Reports: UR354579-UR3: BODIPY-Pd Complexes for Photocatalytic C-C Coupling Reactions
Hongshan He, PhD, Eastern Illinois University
The objective of this project is to test the hypotheses that BODIPY (BODIPY= 4,4'-difluoro-4-bora-3a,4a-diaza-s-indacene) chromophore can activate Pd(II) through an intramolecular electron transfer process for catalytic Sonogashira C-C coupling reaction under visible light illumination. We plan to prepare new BODIPY-modified 1,10-phenanthroline ligands and their Pd(II) complexes to test this hypothesis. The absorption, fluorescence, and electrochemical properties will be determined to reveal the energy transfer process. The theoretic calculations will be performed to provide insights on the energetics of intermediates. Detailed photocatalytic properties of the Sonogashira C-C coupling reactions will be investigated to reveal the reaction mechanism.
In Year 3, one graduate student continues to work on the project and one undergraduate student participated in the project during the summer of 2017. Both students obtained significant training on manipulation of air-sensitive materials sing Schlenk lines and glovebox. The graduate student learned how to use GS-MS and analyze the data. A manuscript is currently under preparation for publication.
As planned, we first configured the reaction condition using the reaction between iodobenzene and phenylacetylene as shown below. All reactions were carried out in DMF at room temperature under nitrogen. Table 1 summarizes the important findings. No product was detected in the absence of catalyst. However, when 5% of catalyst was employed, yield was 92% after 4 hours illumination. If only 0.5% catalyst was used, yield was also 92% after 17 hours. In a stark contrast, the reaction only gave 20% yield under a dark condition. In addition, we performed the same reaction using compound 2, in which no BODIPY moiety was attached. Only 6.6% yield was observed. These results demonstrated clearly the photocatalytic nature of the reaction and key role of BODIPY moieties in the catalysis.
Figure 1. Conventional Sonogashira cross-coupling reactions and structures of photocatalysts for this study
Table 1. Optimization of reaction conditionsa
|
| ||||
Entry | Catalytic system | Yield (%)b | |||
|
|
| 4h | 17h | 24h |
1 | No catalyst | IL | ND | ND | ND |
2 | 1 (5%) | IL | 92 | ~100 | - |
3 | 1 (0.5%) | IL | 18 | 92 | 97 |
4 | 1 (0.5%) | D | 4.2 | 20 | 23 |
5 | 2 (0.5%) | IL | 4 | 6.6 | 5.2 |
a: The reaction conditions: catalyst, phenylacetylene (1.2 mmol), iodobenzene (1.0 mmol), DMF (4.0 mL), PPh3 (0.005 mmol), and Et3N (1.0 mL). Light source: Phillips LED bulb, 26 w. The distance between lamp and the reaction flask was about 10 cm. b: GC yield.
We then studied the reactions between iodobenzene and phenylacetylene with different substituents with 0.5% (mol) of [Pd(BDP)Cl2] in DMF. Table 2 summaries the outcomes from these experiments. The reaction occurred as expected under the similar conditions, however, the yield was quite different. When the R1 was kept as H in phenylacetylene, it was found that varying the R2 in iodobenzene only gave the very low yield with no product being detected after 4 hours for R2 = NO2. Surprisingly, it was found that the yield was high when the R2 group varied from CN, N(CH3)2 to NO2. Therefore, the reaction yield is more dependent upon the structure of iodobenzene then on the phenylacetylene.
Table 2. Yields of different C-C coupling reactionsa
|
| ||||
Entry | Substituents | Yield (%)b | |||
| R1 | R2 | 4h | 17h | 24h |
1 | H | H | 18 | 92 | 97 |
2 | H | CH3 | 17 | 21 | 21 |
3 | H | OCH3 | 3 | 4 | 6 |
4 | H | NO2 | 0 | 8.5 | 18 |
5 | CN | H | 79 | 100 | - |
6 | N(CH3)2 | H | 44 | 92 | 96 |
7 | NO2 | H | 8 | 45 | 64 |
a: The reaction conditions: catalyst, phenylacetylene (1.2 mmol), iodobenzene (1.0 mmol), DMF (4.0 mL), PPh3 (0.005 mmol), and Et3N (1.0 mL). Light source: Phillips LED bulb, 800 lumines. The distance between lamp and the reaction flask was about 10 cm. b: GC yield.
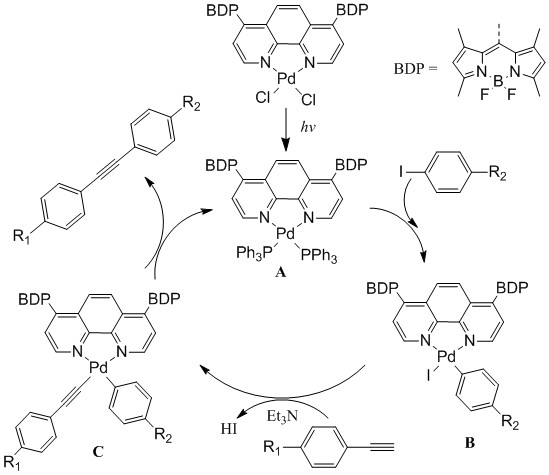
The UV-VIS absorption spectroscopic studies showed under illumination the absorption intensity of Pd-PH1 around 510 nm decreased slowly in the presence of Et3N and PPh3 whereas absorption above 550 increased slightly with an isosbestic point at shoulder peak appeared at 548 nm. Preliminary 31P NMR study and high-resolution mass spectra indicated the coordination of PPh3 to the Pd center after illumination for about 1 hour. A proposed mechanism is depicted in Figure 2. Under illumination, BODIPY moieties facilitated the reduction of palladium from Pd(II) to Pd(0), followed by coordination of PPh3. The rest is quite similar to the conventional Sonogashira reaction except cis-trans isomerization. Phenyl and acetylene groups will bind to Pd(0) in a cis position for easy coupling as shown in Figure 3. Further evidence needs to be acquired to confirm this mechanism.
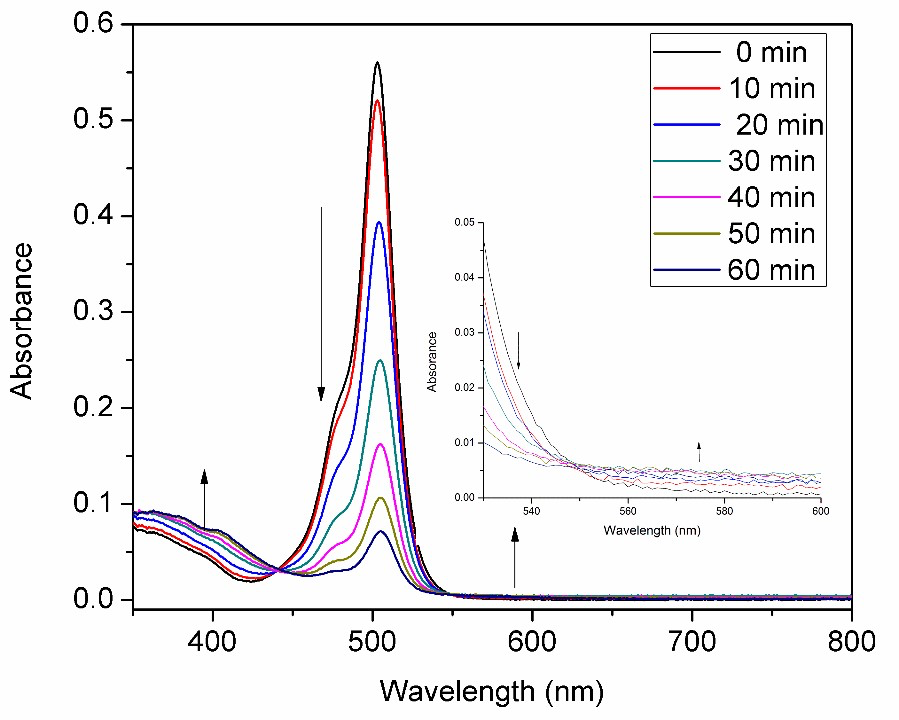
Figure 2. UV-VIS changes of Pd-PH1 with Et3N and PPh3 under illumination in DMF at room temperature.
Figure 3. Proposed photocatalytic C-C cross-coupling reactions between iodobenzene and phenylacetylene