Reports: DNI555946-DNI5: Designing Uniform Paired Copper Catalytic Sites for Conversion of Methane to Methanol
Nicholas Brunelli, PhD, The Ohio State University
The work associated with grant has progressed on several fronts, including organosilane, support synthesis, and some of the overall material characterization, as described in aim 1 and aim 2 of the proposal. Specifically, the aims were:
Aim 1: Demonstrate formation of uniform paired copper sites through synthesis of paired Brønsted acid sites
Aim 2: Demonstrate the ability to alter the distance between paired copper sites through tuning the spatial separation of the acid sites
We have made progress in synthesizing SAPO-5 and SAPO-34 using a paired site precursor that has the potential to create a heterogeneous support with paired Bronsted acid sites and to control the spacing between the paired catalytic sites.
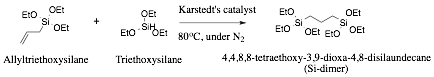
The first step for the overall project involves the synthesis, purification, and characterization of an organosilane that has two silicon atoms with a controlled separation. This was accomplished through synthesis of a precursor with paired silicon atoms separated by a propyl linker. The synthesis involved the hydrosilylation of allyl triethoxysilane with triethoxysilane using Karstedt's catalyst (Scheme 1).
Scheme 1. Synthesis procedure to create paired silicon dimer.
After a reaction time of 18h, the triethoxysilane (added in excess) is distilled off using vacuum distillation as the first cut. The second cut obtained corresponds to the product Si-dimer that is characterized using 1H solution NMR (see figure 5).
Figure 1. 1H NMR for Si dimer with a propyl linker. The peak integration was found to be quantitative.
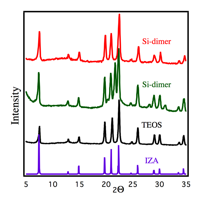
Before synthesis of the SAPO materials with the paired site precursor, we sought to investigate the crystallization of SAPO materials with isolated site precursors in tetraethylortho silicate (TEOS). We chose to start with SAPO-5 (AFI framework) as the microporous materials for our investigation. The AFI framework consists of 12MR as against 10MR for ZSM-5 (MFI framework). Although the ring size of AFI framework is larger, it is important to note that the paired Brønsted acid sites that we wish to generate will only be separated by one T atom (Al) as against 2 T atoms in the case of MFI. Thus, the resultant Cu-Cu distance in Cu-SAPO-5 is expected to be lower than 2.87 Å. Using TEOS, alumina, phosphoric acid, and triethylamine (as the structure directing agent), we were able to crystallize pure SAPO-5 as show in Figure 2.
Figure
2. XRD
for SAPO-5 synthesis using Si-dimer. Purple: IZA reference for AFI. Black: synthesis using TEOS. Red/Green: synthesis using Si-dimer.![]()
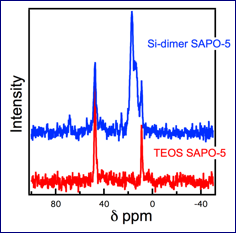
After identifying the conditions to successfully produce SAPO-5, the fully characterized precursor was incorporated in place of the silicon source into the synthesis gel for SAPO-5. The synthesis gel crystallized to create a catalytic material with a paired silicon site. The synthesis preocedure was able to successfully generate a crystalline material with the desired SAPO-5 (AFI) framework. To confirm the presence of the alkyl linker, the material was heated (90°C) under nitrogen atmosphere to get rid of maximum triethylamine present in its pores. The material was characterized using 13C CP/MAS NMR and compared to an uncalcined SAPO-5 sample synthesized using TEOS at 200°C. Comparing the two spectra revealed that there are two other C species present in the SAPO-5 synthesized using Si-Dimer. From our initial investigations, we have been able to use NMR to demonstrate that peaks consistent with the formation of the Si dimer in the material were present, as shown in Figure 3.
Figure 3. Solid state NMR of the SAPO-5 material after crystallization using TEOS (red) and the Si-dimer (blue) as the silicon source.
The alkyl spacer between the paired silicon sites was removed through calcination to and achieve the paired acid site. Crystallizing a material with a novel precursor has required extensive characterization utilizing standard characterization methods (e.g., XRD, nitrogen physisorption, 13C NMR). Thus far, these methods have demonstrated that the material produced is highly crystalline (XRD and N2 physisorption) and contains a paired catalytic site.
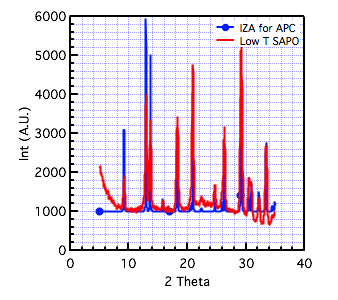
While the material was demonstrated to be highly crystalline, we were also interested in understanding the limits for crystallization to facilitate formation of the crystalline catalytic material. One potentially important parameter affecting crystallization and stability of the material with the dimer could be the temperature. We therefore examined lower crystallizations temperatures of 120°C. While attempting to produce an AFI framework, we determined that an APC framework was produced, as confirmed using XRD shown in Figure 4. This result was significant since previous reports have not demonstrated formation of a SAPO material with an APC framework. We are currently investigating the synthesis conditions that result in formation of APC and the catalytic capabilities of the material.
Figure 4. XRD patterns of SAPO synthesized using TEOS at 120°C with additional water added. The synthesis gel was prepared in a round bottom flask instead of a Teflon reactor. XRD corresponds to APC and not AFI.
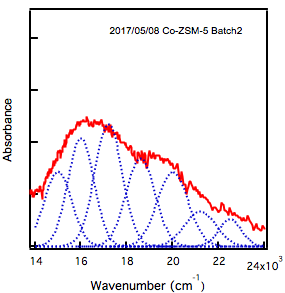
While synthesizing these materials with paired sites has made progress, we are currently extending our characterization to confirm the presence of paired catalytic sites. Recent work in synthesis of MFI (ZSM-5) has demonstrated that exchanging cobalt into the material can be used to demonstrated paired site formation. Therefore, we have synthesized an MFI material using procedures and are beginning to use diffuse reflectance UV-vis spectroscopy to confirm the paired site, as shown below for MFI in Figure 5.
Figure 5. DRUVS of cobalt exchanged MFI.
In addition to this work, the student supported by this grant has made several smaller contributions that will be acknowledged in associated manuscripts – both published and upcoming. First, the student has synthesized dimeric tin complexes that can be incorporated into synthesis of heterogeneous SnAPO materials that can provide heterogeneous materials with different acidity. Second, the student has been involved with finalizing a project that an undergraduate initiated in our research. The project involved designing heterogeneous catalytic materials using strengths of the student in organosilane synthesis. This work has resulted in a published manuscript that acknowledged ACS-PRF for financial support. Finally, the student has been involved in synthesis of Sn-BEA and Sn-MFI materials for alcohol ring opening of epoxide. This work will acknowledge financial support from ACS-PRF.