Reports: UNI955668-UNI9: Kinetic Studies to Optimize Chemical Dissolution and Inhibition of Common and Exotic Oilfield Scales
Jessica M. Wilson, PhD, Manhattan College
Hossain Azam, PhD, Manhattan College
Project Objective and Background
The objective of this research is to optimize the conditions for using chelating agents/phosphonate based chemicals to dissolve and inhibit the exotic oilfield scales SrSO4, ZnS and PbS. Inhibition and dissolution of these mineral scales are minimally studied. Thus, chemical dissolution and inhibition of these scales, which significantly decrease well production and damage equipment, can ensure continued oil well production.
This progress report presents the following:
1) Optimal chelating agent concentration for the dissolution of the mineral scales under different temperatures and determination of rate constants for the dissolution under optimal conditions.
2) Identification of effective phosphonate-based chemical for inhibition of ZnS and PbS.
Work Conducted
1) Dissolution of SrSO4
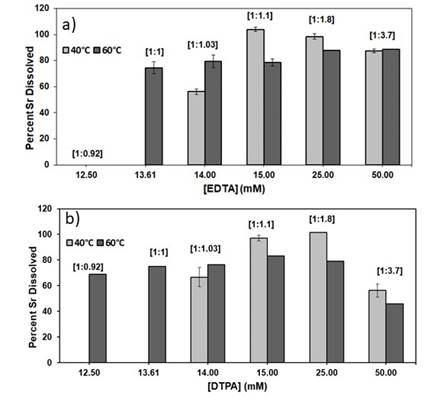
24 hr experiments were conducted to determine maximum extent of SrSO4 dissolution in presence of different concentrations of the individual chelating agents (EDTA and DTPA) and temperature at pH 12 as shown in Figure 1. Once the optimal chelate concentration was determined as 15 & 25 mM EDTA and DTPA, kinetic experiments were conducted in duplicates for 3 hours to determine the rate of dissolution at pH 12 at 40°C as shown in Figure 2.
Figure 1. Percent Sr2+ dissolved at different concentrations of a) EDTA and b) DTPA at t = 24 hr, pH = 12, T = 40ºC, 60ºC. Error bars represent relative percent difference (RPD) between duplicates. The metal to ligand mole to mole ratio [Sr2+:L] is shown at the top of each bar.
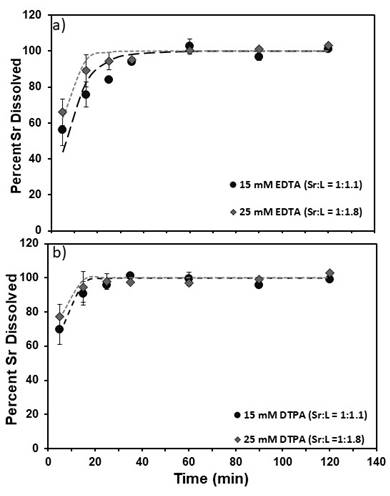
100% dissolution was achieved after 1 hour for both EDTA and DTPA at both concentrations at 40°C. The observed first-order reaction rates were calculated in hr-1 and are shown in Table 1. The average dissolution rate of SrSO4 in presence of DTPA is faster than the rate in presence of EDTA at both concentrations.
Figure 2. Percent Sr2+ dissolved versus time (min) for a) EDTA (15 mM and 25 mM) and b) DTPA (15 mM and 25 mM) at T=40ºC, pH = 12. Also shown on plot are first-order model fits (dashed lines). The metal to ligand mole to mole ratio has been shown in the legend.
Table 1. Observed first order rate constants for the dissolution of SrSO4 with EDTA and DTPA at 40ºC.
Dissolver | kobs, 40ºC (hr-1) |
15 mM EDTA | 6.96 ± 2.07 |
25 mM EDTA | 11.94 ± 3.95 |
| |
15 mM DTPA | 13.56 ± 2.96 |
25 mM DTPA | 17.39 ± 5.15 |
2) Dissolution of ZnS and PbS
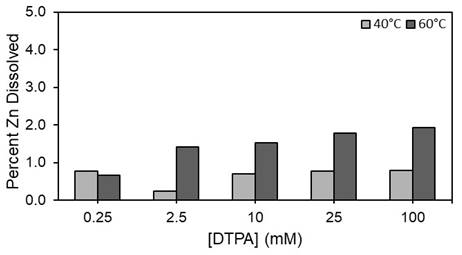
Dissolution experiments were run for 24 hours at different ligand concentrations and temperature at pH 12 and the overall percent dissolution of ZnS and PbS were determined in terms of free metal concentration. The effects of different chelate concentrations on the dissolution efficiency of the ZnS scale are shown in Figure 3 for DTPA.
Figure 3. Percent Zn2+ dissolved at different concentrations of DTPA at t = 24 hr, pH = 12, T = 40°C, 60°C.
The maximum dissolution found was less approximately 2% at 60°C for 24 hrs, and approximately 6% after 175 hrs indicating that DTPA (data shown) and EDTA (data not shown) is not effective at dissolving ZnS (data shown) and PbS (data not shown). Application of the previously discussed kinetic model to this data resulted in a dissolution rate of ZnS that is 10000 times slower than SrSO4.
3) Inhibition of ZnS and PbS
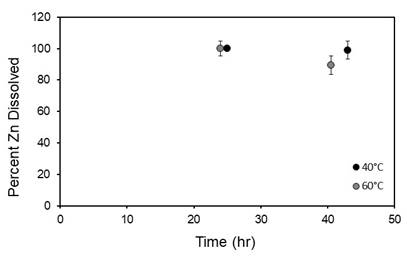
As the chelating agents EDTA and DTPA were found to not be effective at dissolving ZnS or PbS, an alternative chemical approach is to use a phosphonate based inhibitor to inhibit scale formation. NTMP was used as the inhibitor where [Zn:NTMP] = [1:1]. A 45 hour inhibition experiment was conducted at 40°C and 60°C and pH 6 to assess the effectives of NTMP to inhibit ZnS formation. The dissolved Zn concentration was tracked and results show 88% to 100% inhibition for both temperature (Figure 4), indicating that NTMP is effective at inhibiting ZnS formation.
Figure 4. Percent Zn2+ dissolved versus time (hr) for [Zn:NTMP] = [1:1] at T = 40°C, 60°C.
Future Work
Additional work is being conducted to determine the optimized SrSO4 dissolution reaction rates at other temperatures and pH and to determine the inhibition of SrSO4. After the completion of the rest of dissolution and inhibition experiments on SrSO4, ZnS and PbS, dissolution and inhibition results will be utilized to observe the performance of optimized chelating agent types, concentration with optimal pH and temperature on real life oilfield produced water.
Broader Impacts
This research has had significant impacts for the PIs as well as their research students. This has influenced their careers as they have expanded their research scopes. The PIs have supported three undergraduate students and two graduate students through this funding. Table 2 outlines the students and their specific outcomes of their contributions to this to this work. As shown in Table 2, contributing to this research has sparked two undergraduate students’ interests in pursuing graduate work. Contributing to this work will greatly benefit all students as they pursue careers in environmental engineering and chemistry after graduation.
Table 2. Student researchers and outcomes.
Student/Level Major and Graduation Date | Outcome |
Fiona Dunn/Senior BS, Civil Engineering, May 2017 | · Currently a PhD student at New York University · Presented at on campus research symposium Sept. 2016 |
Goldie Gunawan/Senior BS, Civil Engineering, May 2017 | · Currently MS student at University of Michigan · Presented at on campus research symposium Sept. 2016 |
Stephanie Castro/Graduate Student ME Env. Engineering, Dec. 2017 | · Presented at National American Chemical Society Conference, Washington, DC, Aug. 2017 · Awarded 2nd Place (with Ed Horai) Platform Presentation at New England Graduate Student Water Symposium, Amherst, MA, Sept. 2017 |
Ed Horai/Graduate Student ME Env, Engineering, Dec. 2017 | · Presented at National American Chemical Society Conference, Washington, DC, Aug. 2017 · Awarded 2nd Place (with Stephanie Castro) Platform Presentation at New England Graduate Student Water Symposium, Amherst, MA, Sept. 2017 |
Daniela Coll de Pasquali/Sophomore BS Chemical Engineering, May 2019 | · Presented at National American Chemical Society Conference, Washington, DC, Aug. 2017 · Presented at on campus research symposium, Sept. 2017 |