Reports: DNI756823-DNI7: Resolving the Impact of Mesoscopic Heterogeneities on the Transport Properties of Gels
Chaitanya K. Ullal, PhD, Rensselaer Polytechnic Institute
Polymeric microgels, gel particles that have sizes that span from the meso to the nanoscale, constitute a relatively new class of colloids. When compared to their bulk counterparts, microgels exhibit much higher specific surface and swifter swelling response. Poly(N-isopropylacrylamide) (PNiPAm) pervades the literature as a thermoresponsive constituent of microgels. Of interest to this project is the fact that the network structure and morphology of PNiPAm microgels is strongly dependent on reaction conditions. Efforts in the first year of the project have centered on controlling the spatial variations in crosslink density of PNiPAm microgels while incorporating crosslinkers that can be functionalized via click chemistry.
Click-functionalizable crosslinker
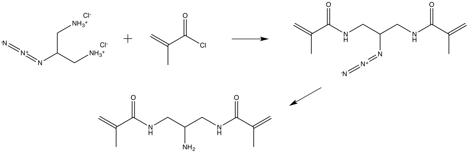
The stereotypical crosslinker used in the synthesis of PNiPAm microgels is methylene-bis-acrylamide (BIS). The guidelines for the design of the functionalizable crosslinker were set as follows: i) molecular dimensions, composition and physicochemical behavior equivalent to BIS ii) compatibility with free radical polymerization reactions in aqueous media and iii) pendant functionality for the facile chemical derivatization with dye molecules. To this end, we designed and synthesized a novel functional crosslinker, N,N'-(2-aminopropane-1,3-diyl)bis(2-methylacrylamide), DNH2 for short, where the pendant functional group is a primary amine. First we synthesized the previously reported 2-azidopropane-1,3-diaminium chloride1. Next, the salt was converted to the free amine in dichloromethane using 10x equivalents of triethylamine, followed by acylation with methacryloyl chloride. The compound was purified with silica gel flash chromatography using ethyl acetate as the eluent. Finally, DNH2 was synthesized by reducing the azide group with tin chloride dihydrate in MeOH and purification with silica gel flash chromatography using a dichloromethane/methanol 85/15 mixture.
Figure 1. Synthetic route developed for the click-functionalizable crosslinker N,N'-(2-aminopropane-1,3-diyl)bis(2-methylacrylamide).
To verify the covalent incorporation of the click-functionalized crosslinker into the polymer network, PNiPAm hydrogels were synthesized with 1:500 molar ratio of Rhodamine functionalized crosslinker with respect to BIS (0.2% DNH@Rh). As a control, hydrogels containing the same molar fraction of free Rhodamine dye were also synthesized under the same conditions. Both hydrogels were submerged in water. As can be seen in Figure 2, the Rhodamine in the covalently linked hydrogel samples did not leach, while the control samples did.
Figure 2. Leach test demonstrating covalent incorporation of dye tagged crosslinker into N-Isopropylacrylamide hydrogels. Left: control sample. Right: covalently tagged crosslinkers.
Microgel synthesis: homogeneous via feed versus heterogeneous via batch
Conventional free-radical precipitation polymerization in water was employed for the preparation of PNiPAm microgels with heterogenous and homogeneous crosslinking density. The notion of structural heterogeneity was identified as early as the first reports on PNiPAm microgels and is ascribed to the unequal reactivities between NiPAm and BIS2. One established path for the preparation of homogeneously crosslinked particles is the imposition of monomer-starved conditions3. Here, monomers are fed into the reactor at the rate of consumption, effectively maintaining a constant molar ratio between the monomers at any given instance. Since one of the comonomers is the crosslinker, this results in homogeneous crosslink distribution. All other synthetic parameters were kept identical for the two types of microgels. The amount of functional crosslinker was kept sufficiently low to avoid possible perturbation of the microgel nanostructure but still retaining particle fluorescent brightness.
Figure 3. Reaction setup
Impact on network morphology measured through dynamic light scattering
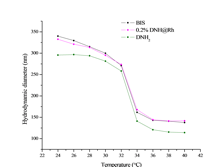
Using variable temperature dynamic light scattering we examined the effect of the incorporation of dye-tagged crosslinker molecules into microgels on the following properties: i) hydrodynamic dimensions, ii) dispersity and iii) swelling ratio and iv) volumetric phase transition temperature. From the cumulative of those results it is possible to derive information related to the uniformity of crosslink distribution in the microgel network. Experiments in which BIS was completely substituted by the precursor aminated functional crosslinker were also conducted. Preliminary results for inhomogeneous microgels are presented in Fig. 4. The samples were monodisperse with values below 0.1 at the measured temperature set. The size, thermal response and phase transition temperature of dye tagged microgels (0.2 mole% DNH@Rh with respect to BIS) are practically indistinguishable from those of the pristine microgels (BIS). This behavior validates the use of the dye tagged crosslinker molecules as reporter agents of the crosslinking density distribution of PNiPAm microgels, at least at loadings as low as 0.2%. The fully aminated crosslinker microgels, show a symmetric shift of the hydrodynamic dimensions towards lower values, while the swelling ratio remains relatively unaltered, a feature denoting comparable particle stiffness and concurrently mesh size characteristics. The smaller size suggests that the aminated crosslinker affects the initial stages of particle formation. In this light, the functional crosslinker molecule provides an additional handle for the regulation of particle size, whilst the pendant groups can serve as anchors for post-modification reactions.
Figure 4. Hydrodynamic diameter versus temperature of PNiPAm microgels polymerized with BIS (blue), 0.2 mol% dye tagged crosslinker incorporated into the BIS (pink), and aminated crosslinker (green). The introduction of 0.2 mol% dye tagged crosslinker does not perturb the properties of the microgel particles.
Impact on students and career.
Already in the first year, the DNI funding has had a strong impact on the career trajectory of the PI. The receipt of the award was rated highly in the PI's third year evaluation and it also facilitated the award of associated funding from the NSF. The funding led to the hire of a post-doc with significant experience in the material systems being investigated. This has accelerated the productivity in this research area. The post-doc has also served as a mentor to a talented undergraduate student on the project.
References
1. Urankar, D.; and Kosmrlj, J.; Preparation of diazenecarboxamide - carboplatin conjugates by click chemistry, Inorganica Chimica Acta 363 (2010) 3817 - 3822.
2. Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods D.R.; and McPhee W.; The kinetics of poly(N-isopropylacrylamide) microgel latex formation, Colloid and Polymer Science 272 (1994) 467 - 477.
3. Acciaro, R.; Gilanyi, T.; and Varga. I.; Preparation of Monodisperse Poly(N-isopropylacrylamide) Microgel Particles with Homogenous Cross-Link Density Distribution, Langmuir 27 (2011) 7917 - 7925