Reports: ND555955-ND5: Gaining Mechanistic Insight in Heterogeneous Catalysis at the Single Molecule Level: Orbital Imaging of a Macromolecular Catalyst and Intermediates by Scanning Tunnelling Microscopy
Sarah A. Burke, PhD, University of British Columbia
The first year of this ACS PRF project has been focused on building up tools and capabilities to explore catalytic activity of our self-assembled macromolecular iron-terpyridine (Fe-tpy) structures by scanning probe microscopy (SPM) methods. We have also continued to work on gaining knowledge of the basic structure and electronic structure of this complex as a baseline to understand reactivity.
Understanding the structure and electronic states of nascent macromolecular iron-terpyridine structures on Ag(111)
Data acquired on the ligand used to assemble the Fe-tpy macromolecular catalyst species on-surface were fully analyzed to understand the interaction between the active tpy functional groups and the metallic substrate used as a support. High resolution imaging confirmed that the outer/distal pyridines of the tpy structure are pointing away from the coordination site with implications both for intermolecular interactions and coordination. Further, a specific orientation of the molecule with respect to the silver substrate was found, indicating a relatively strong interaction. Most interestingly, scanning tunneling spectroscopy (STS) measurements on the bare Ag(111) compared to those on a decoupling bilayer of NaCl indicate that the unoccupied orbitals associated with the tpy groups are strongly hybridized with the substrate, driving a strong substrate interaction as well as changes in the electronic structure. This emphasizes that the whole system needs to be taken into account, including the substrate, when considering molecules with functional groups that strongly interact with metals like those used in coordination bonding driven networks. This work is in revision for the Journal of Physical Chemistry with 5 former trainees as co-authors.
As a baseline for understanding the electronic structure and reactivity of the Fe-tpy macromolecular structures on the surface, we have continued to investigate the as-formed nascent metalorganic species. We now have convincing evidence from STS data of Fe states just below the Fermi energy, which should contribute to the reactivity of these complexes. There remain open questions concerning the detailed structure of the Fe atoms in the linkages between ligand molecules. This work is an ongoing collaboration with my former postdoctoral researcher Dr. Agustin Schiffrin who has started his independent career at Monash University, who in addition to applying SPM-based techniques to attempt to resolve these questions, has also applied synchrotron XPS and NEXAFS measurements. Prior to her final dissertation submission, my former student Dr. Martina Capsoni participated in these experiments supported by this ACS PRF grant giving her experience with techniques beyond SPM. Despite the application of these multiple techniques in conjunction with density functional theory calculations the exact structure of the linkage containing either two or three iron atoms in an approximately linear configuration still contains some uncertainty. As the starting structure of the catalyst is an important piece of information for understanding the reactivity, we will continue to investigate the nascent catalyst and seek conclusive evidence for one of the possible structures, or if multiple structures are present. Interestingly however, the reaction of the complex with different reactants may provide further clues as to the underlying structure. To this end, we are also directly embarking on the experiments designed to probe reactivity and reaction mechanisms of small hydrocarbon molecules with this metal-organic centre.
Building capability for exploring reactivity
A controlled gas dosing system was designed and constructed in the first four months of the project (May-Aug 2016) as an undergraduate summer research project. This entailed building a gas handling system for individually dosing different gasses into the ultrahigh vacuum low temperature SPM chamber. To enable introduction of different gasses to explore reactivity, the system includes the ability to fully pump out each gas and introduce a new gas to the lines leading to the high precision leak valve that allows calibrated controlled dosing at ultrahigh vacuum pressures. A nozzle was also constructed to direct the flow of gas into the ultrahigh vacuum chamber so that the gas is dosed more locally and directed towards the low-temperature SPM where the sample resides. This system was installed in Sept 2016 and is also being used to dose samples with CO molecules to allow for tip functionalized nc-AFM measurements as described below.
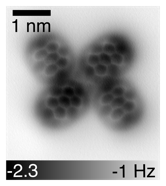
Figure 1: constant height CO-functionalized nc-AFM image of a PTCDA quatramer demonstrating clear submolecular resolution to be used on nascent and reacted Fe-tpy macromolecular structures.
Tip-functionalized nc-AFM measurements have become an increasingly important compliment to STM measurements for obtaining exquisitely detailed structural information about adsorbed molecules. This method, where a single CO molecule is attached to the tip via vertical manipulation, provides clear images of the bonds, including information linked to bond order, rather than the electron density near the Fermi energy. This has been demonstrated by several others as being a powerful tool for the identification of uncertain molecular structures. Over the past year we have been developing expertise in this technique in-house, aided by the above gas dosing system to introduce CO molecules for the tip functionalization. As a starting point to build up this expertise, we applied this to a well-known molecular species that we had previously investigated by STM. Figure 1 shows a quatramer of 3,4,9,10-perylene tetracarboxylic dianhydride (PTCDA) on an NaCl bilayer on Ag(111) imaged by nc-AFM with a CO functionalized tip clearly showing the 5 rings of the pyridine core (the oxygen atoms at the ends of the molecule are not resolved as they are closer to the surface and carry different charge). With this tool in hand, we will be able to a clearer view of the nascent complex as well as where reactants attach to the complex and what products are formed and remain on the surface.
In addition to this progress, there was significant trainee turnover in the group. New trainees have been trained in the operation of the complex instrumentation and techniques required over the past year, carried out preliminary experiments, and now have the expertise to carry out the proposed experiments. These were scheduled to start running full-time in spring 2017, however, due to a major equipment failure resulting in 5 months downtime, these are just underway now.