Reports: UNI456726-UNI4: Sulfur Chemistry: Molecular Mechanisms
AnGayle Vasiliou, PhD, Middlebury College
A. Major Activities
We have studied the unimolecular decomposition of ethanethiol and thiophene. We have established direct evidence of radical intermediates formed during thermal decomposition. Experiments were done with the use of a hyperthermal nozzle configured to matrix isolation infrared absorption and vacuum ultraviolet photoionization mass spectroscopies. These techniques both allow for thermal tuneablity (298 K - 1700 K) and sensitive detection of intermediate species such as radicals. All experiments were done in an anaerobic environment.
Ethanethiol:
Our experiments have enabled us to identify the initial thermal decomposition products are ethanethiol. We have shown that ethanethiol thermal cracks into three distinct pathways:
We have published our findings in the scientific literature.
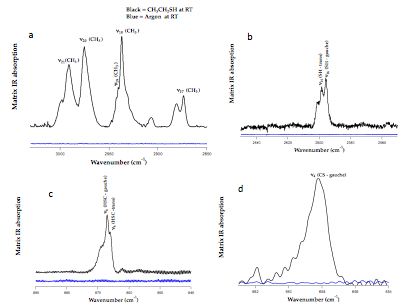
As we have determined a detailed mechanism for the thermal decomposition of ethanethiol we are currently working on providing vibrational assignments for both the gauche and trans conformer of the molecule. Figure 4 shows the infrared spectrum of ethanethiol taken with the matrix isolation apparatus. A mixture ratio 0.00632% ethanethiol to argon was used for matrix IR experiments. The spectra in Figure 1 depicts four unique regions of the IR: a) CH stretching region b) SH stretching region c) and d) bending regions for sulfur species. Figure 1 demonstrates that all regions with active modes can be detected and assigned with our experimental apparatus. The data in Figure 1d is also proof of concept that we have the capability and resolution to distinguish between different stable conformers of a molecule, such as the gauche and trans (or anti) form of ethanethiol. Although ethanethiol has been previously studied in the IR, the limitations of the experiments could not resolve the two conformers. As a result, several peaks were left unassigned or generically assigned as an "SH stretch" rather than as "SH-gauche" stretch. Due to the lack of experimental data, there is discrepancy among mode assignments in the current literature. To our knowledge, our study will be the first experimental study to elucidate the vibrational assignments of both gauche and trans ethanethiol. A complete list of experimental assignments with preliminary calculations is shown in Table 1. As shown in Table 1 we have tentatively assigned several of the trans modes. Geometry optimizations and frequency calculations were performed at B3LYP/6-31G** level of theory. To date in the literature only two trans modes have been experimentally observed: v16 and v19 In addition to taking spectra in an argon matrix we plan to use neon as the inert gas, which known to sharpen features in IR spectra.
Figure 1: Matrix IR spectra of Ethanethiol. A selection of modes: a) CH stretching region b) SH stretches c) HSC bending modes d) the CS bend
Table 1: Preliminary experimental and calculated vibrational frequencies for ethanethiol form PI's lab
Mode |
Approximate Description |
Exp. (cm-1) |
Calc. (cm-1) |
||
gauche |
trans |
gauche |
trans |
||
v1 |
CH3 sym str. |
2991 |
2956 |
3093.1 |
3097.5 |
v2 |
CH3 asy str. |
2976 |
2952 |
3115.4 |
3114.8 |
v3 |
CH2 str. |
2941 |
2943 |
3085.0 |
3090.1 |
v4 |
CH3 asy str. |
2937 |
2907 |
3057.4 |
3058.2 |
v5 |
CH2 str. |
2873 |
2881 |
3026.9 |
3034.6 |
v6 |
SH str. |
2598 |
2596 |
2671.3 |
2672.8 |
v7 |
CH3 asym def. |
1459 |
1457 |
1500.5 |
1505.8 |
v8 |
CH3 asy def. |
1452 |
1492.7 |
1496.0 |
|
v9 |
CH2 def. |
1437 |
1439 |
1480.6 |
1492.8 |
v10 |
CH3 sym def. |
1379 |
1415.9 |
1418.8 |
|
v11 |
CH2 wag. |
1277 |
1274 |
1307.9 |
1300.9 |
v12 |
CH2 twist |
1252 |
1260 |
1282.3 |
1270.7 |
v13 |
CH3 rock. |
1097 |
1099 |
1121.4 |
1110.5 |
v14 |
CH3 rock. |
990 |
1063.3 |
1045.4 |
|
v15 |
CC str. |
966 |
975 |
980.2 |
990.2 |
v16 |
CSH bend. |
870 |
869 |
875.4 |
858.1 |
v17 |
CH2 twist |
1252 |
1260 |
1282.3 |
1270.7 |
v18 |
CH3 rock. |
990 |
1063.3 |
1045.4 |
|
v19 |
CH2 rock. |
733 |
730 |
736.3 |
792.4 |
v20 |
CH3 torsion |
Not obs. |
Not obs. |
255.1 |
248.9 |
v21 |
SH torsion |
Not obs. |
Not obs. |
214.0 |
161.5 |
Thiophene:
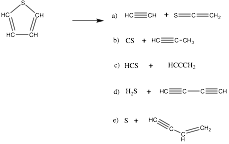
Our experiments and theoretical work have enabled us to determine the thermal decomposition mechanism for thiophene. We have shown that ethanethiol thermal cracks into five distinct pathways:
The reactions were monitored with matrix isolation spectroscopy and VUV photoionization mass spectrometry. The experimental data collected were explained by the unimolecular decomposition of thiophene to its pyrolysis products. CBS-QB3 calculations were used to interpret the experimental results and to visualize the decomposition pathways. We have submitted and have had our findings accepted in the scientific literature.
B. Student Impact
In the last year, five undergraduate researchers have carried out this work under the supervision of the PI, working full time during the summer and roughly 10 hours per week during one or more academic year semesters. Three of the five have been have been from under-represented groups and one matriculated into graduate school in chemistry this fall 2017. The goal of getting the students involved in this type of research experience is to encourage them pursue careers in the STEM fields. As the PRF project has both experimental and computational aspects it exposes students to both sides of physical chemistry. Undergraduate have learned to work with sophisticated lab equipment such as turbo pumps, cryogenics and lasers. In addition, students have been mentored and how to use the scientific literature to explain their findings as well as present their finding to a variety of audiences. The undergraduates give oral presentations at the Research Symposium, poster presentations at the Middlebury College Summer Research Symposium, and as co-authors on presentations at national meetings and manuscripts.
C. Scientific Impact
Our results on both ethanethiol and thiophene have been published in the scientific literature. Additionally, our results will be presented at the Summer National Meeting of the American Chemical Society in Boston, MA in August 2018. The PI has also given invited lectures on PRF research at Wesleyan University (Spring 2017) and the University of Richmond (Fall 2018)