Reports: ND755503-ND7: Development of New Liquid Crystalline Polyamides with Bicyclo[8.8.8] Hexacosane Flexible Segments
Eugene A. Mash, PhD, University of Arizona
Objective
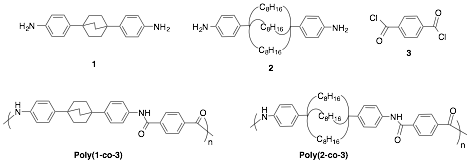
The objective of this research project is the development of new thermotropic liquid crystalline main chain polyamides that incorporate in the polymer backbone large-ring bicyclic groups designed to serve as molecular springs to impart silk-like elasticity to the material. The specific aims of this study are (1) preparation of monomers 1 and 2, (2) their co-polymerizations with terephthaloyl chloride (3) to afford the polymers poly(1-co-3) and poly(2-co-3), and (3) comparisons of the structural, physical, and mechanical properties of these polymeric materials with each other and with Nylon 6-T as a control. The necessary monomers are being prepared on multi-gram scales by adaptations of known synthetic methods. Polymers will be prepared using standard condensation methods. Soluble polymers will be cast into thin films (or fibers) and characterized by wide angle XRD (to determine crystallinity), differential scanning calorimetry (to ascertain glass transition temperatures and melting points), and stress strain analyses. The elastic elongation (before yield point) will be compared with Nylon 6-T to determine if the cyclic and bicyclic structures provide additional elasticity relative to existing polyamides.
Figure 1. Bicyclic diamine monomers 1 and 2 are depicted along with co-monomer terephthaloyl chloride (3) and the copolymers, poly(1-co-3) and poly(2-co-3).
Progress to Date
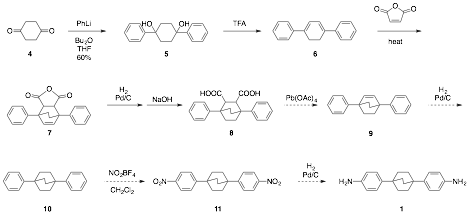
The synthesis of monomer 1 is depicted in Scheme 1. Progress to date is indicated by solid arrows, work to be completed is indicated by dashed arrows. The synthesis is progressing on a large scale, and should deliver multi-gram quantities of 1 for polymerization.
Scheme 1. Synthesis of Monomer 1 (work in progress).
The synthesis of monomer 2 will follow the method depicted in Scheme 2. Progress to date is indicated by solid arrows, work to be completed is indicated by dashed arrows. The synthesis is progressing on a large scale, and should deliver multi-gram quantities of 2 for polymerization.
Scheme 2. Synthesis of Monomer 2 (work in progress)
Project Significance and Potential for Further Development
The innovation of this research involves the introduction into the polymer backbone of large, conformationally mobile cycloaliphatic groups with the proper shape and sufficient to allow the formation of desirable liquid crystalline morphologies for strength, but at the same time with sufficient flexibility to respond as molecular springs distributed within the macromolecular backbone upon the application of tension. We have identified a previously unknown family of monomers, namely, functionalized bicyclo[m.m.m]alkane derivatives that possess large rings. Polymers incorporating these monomers should possess film- and fiber-forming properties and lead to materials that should exhibit substantial reversible elongation along the chain axis while maintaining a high tensile strength. Such polymers would be valuable in the manufacture of shock-resistant cords and durable materials.
This project is the vehicle through which third-year doctoral graduate student Yegor Timofeyenko is learning laboratory practice in synthetic organic chemistry, polymer synthesis, and characterization.