Reports: ND1052309-ND10: Exploratory Synthesis and Characterization of Pyrochlore Oxynitrides for Solid State Lighting
Robin T. Macaluso, PhD, University of Northern Colorado
Mas Subramanian, PhD, Oregon State University
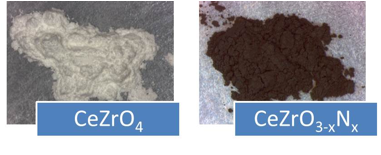
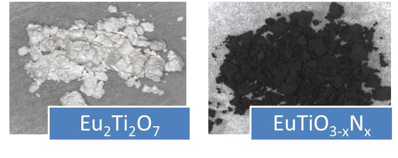
Major accomplishments include the successful syntheses of EuTiO3-xNx and CeZrO3-xNx. Similar procedures were used to make both materials. Eu2Ti2O7 and CeZrO4 were synthesized and then used as precursor materials for ammonolysis (heat treatment under flowing NH3 gas). The resulting oxynitride samples were then reoxidized by heating them under flowing O2 gas. Figure 1 shows optical images of a) the CeZrO4 precursor oxide, b) the CeZrO3-xNx oxynitride, c) Eu2Ti2O7, and d) EuTiO3-xNx oxynitride.
CeZrO3-xNx
Molly Anderson, a graduate student in Macaluso's research group collected neutron diffraction data through the Mail-in Program using the POWGEN beamline at the Spallation Neutron Source, Oak Ridge National Laboratory. Data were collected from three samples – the precursor oxide, CeZrO4; the oxynitride; and an oxynitride sample that was reoxidized under oxygen atmosphere. She collaborated with Dr. Jun Li, a postdoctoral researcher in Prof. Mas Subramanian's group, to analyze the data. LeBail refinements indicate a unit cell expansion of 4.3%, which suggests that nitrogen substitution of oxygen indeed took place during ammonolysis. Thermogravimetric analysis experiments on the oxynitride sample showed a 2.3% weight increase when the oxynitride sample was heated under flowing O2. This weight increase corresponds to CeZrO2.48N0.32.
EuTiO3-xNx
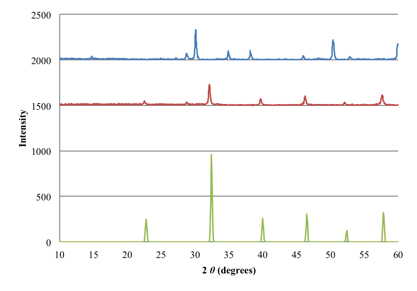
Powder X-ray diffraction patterns collected from EuTiO3 and EuTiO3-xNx samples are shown in Figure 2. The shift of the PXRD peaks from the EuTiO3-xNx sample indicates a unit cell expansion and suggests that nitrogen substitution of oxygen was successful. The sample can be reoxidized to form EuTiO3 as suggested by the shift in smaller 2-theta of the characteristic diffraction peaks (shown as green). Thermogravimetric and combustion analyses also confirm the presence of nitrogen in the oxynitride, EuTiO3-xNx. Neutron diffraction experiments would provide further evidence for the successful synthesis of an oxynitride and further insight into the crystal structure. Powder neutron diffraction experiments of EuTiO3-xNx will be performed in the next academic year.
Figure 7. PXRD patterns of Eu2Ti2O7 before and after ammonolysis, and the known perovskite EuTiO3. Blue, red, and green lines represent Eu2Ti2O7, EuTiO3-xNx, and EuTiO3, respectively. Data for EuTiO3 obtained from (Brous, Fankuchen, & Banks, 1953).
Neither CeTiO4 nor Eu2Zr2O7 successfully yielded an oxynitride under ammonlysis conditions.
A UNC undergraduate student, Linda Nguyen, and Molly Anderson performed exploratory synthesis of oxynitrides using the citrate method, which involves the preparation of an amorphous oxide followed by ammonolysis. An amorphous sample of La2Zr2O7 was successfully synthesized and heated under flowing NH3 gas for six hours at 900 C. PXRD patterns of the sample showed that the sample progressively crystallized with increased heatings; however, the characteristic diffraction peaks did not shift in 2-theta. This suggests that nitrogen substitution of oxygen was not successful using the amorphous oxide.
In the next year, we will:
1) measure the optical properties of EuTiO3-xNx and CeZrO3-xNx
2) measure the electrical properties of EuTiO3-xNx and CeZrO3-xNx
3) collect neutron diffraction data on EuTiO3-xNx
4) prepare a manuscript for publication.
5) continue soft chemistry synthesis efforts. We will explore the effect of various ammonolysis parameters on the synthesis of oxynitrides. Some of the parameters that we will investigate include temperature, dwell time, and placement of the sample relative to the NH3 inlet position.