Reports: DNI1053493-DNI10: Understanding Gas Reactivity at an Interface Using Petroleum Products
Aaron P. Esser-Kahn, PhD, University of California, Irvine
Research in my group in the last two years has evolved to follow two lines of inquiry. First, we are focused on advancing energy application, specifically carbon capture, using microvascular materials.
A. Micro-vascular and designer materials for mass exchange. First, our work in microvascular materials has expanded. We have published within two or three areas focused within carbon capture and sequestration. Currently, we have published six articles in this area, with two more submitted and four more completed projects planned for submission. Our work has been divided among building microvascular materials for enhancing mass transfer and looking at the fundamentals of micro-vascular design. In addition, we have expanded in two areas. First, we are developing a cooperative CO2 binding liquid. We seek to mimic the properties of hemoglobin to capture and release CO2 with a sigmoidal binding isotherm. To accomplish this, we have begun using molecular self-assembly to guide CO2binding. In addition, we have published work using the photo-thermal effect of nanoparticles to regenerate capture solvents. Particularly exciting is the performance of high temperature chemical reactions in low temperature bulk solution by this ultra-localized heating phenomenon. We plan to continue researching and expand this area within the coming years.
In the coming years, we plan to continue these lines of inquiry with three central themes; (1) microvasculature, (2) cooperativity, and (3) photo-thermal heating effects with the goal of improving mass and heat transfer.
Microvasculature:
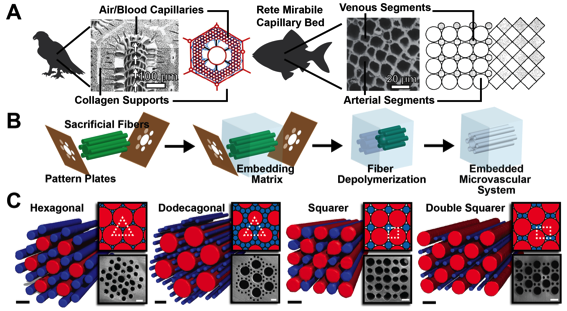
Patterning - My group is interested in patterning and manipulating microstructure for the purposes of creating a “lung”-like structure that captures CO2. We view this as a general experimental platform for understanding and manipulating microstructure. Micro-structured materials have advanced the fields of heat exchange, chemical analysis, flow chemistry, fuel cells, and carbon capture, and are currently advancing the field of tissue engineering. Alongside human-made exchangers, biological systems developed micro-patterned exchange designs for much of evolutionary history. Evolution repeatedly converged on vascular exchange units composed of arrays of circular channels, known as “rete mirabile”. Remarkably, in the 1970s, it was discovered that these bio-exchange designs are the exact solutions to the compact, packing of circles of two diameters. Despite strong interest in micro-exchangers and their many applications, very little work examines biologically inspired systems composed of the close-packed circular microchannels. We studied the transfer characteristics of microvascular exchange units adapted from the solution of close-packed patterns of circles of two diameters. We found that the patterns’ exchange coefficients vary and that biological systems generally employ the more efficient patterns. However, two patterns have not been found in biological systems and yet we found they are also efficient for both heat and mass transfer.
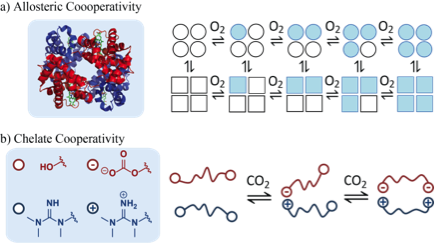
Cooperative CO2 Binders - Looking again to the lung, we developed a small molecule cooperative binder of CO2 instead of oxygen. We recently developed a small molecule system that binds CO2 cooperatively. Designing materials that exhibit cooperativity has been an important, but elusive goal in the field of CO2 capture and sequestration. In CO2 capture, a material must bind CO2 selectively from a mixture of gases, then release the CO2 elsewhere as a purified stream. Cooperativity allows a material to transition between high and low-affinity binding, so that CO2 is strongly bound during the capture phase and easily released during the regeneration phase. Nature employs cooperativity to capture and release its most important gas: oxygen. We sought to mimic the function of hemoglobin, but with CO2as the substrate rather than oxyge.
Recently, we demonstrated that chelate cooperativity emerges from a simple, small-molecule system comprising a bidentate alcohol and a bidentate guanidine. In particular, we show how a key parameter governing the chelate effect, the Effective Molarity (𝐸𝑀), can be measured and controlled by changing solvent conditions. Additionally, we report thermodynamic measurements suggesting that the chelate effect becomes stronger as the entropic cost of CO2 binding (in the absence of the chelate effect) becomes larger.
Figure 1
Figure 2