Reports: UNI352224-UNI3: Nickel Catalyzed C-H Arylation using C-O Electrophiles
Dipannita Kalyani, PhD, St. Olaf College
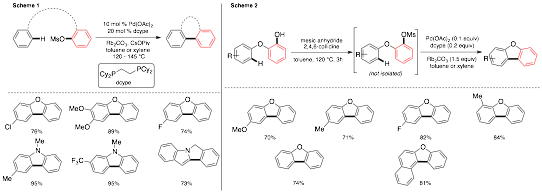
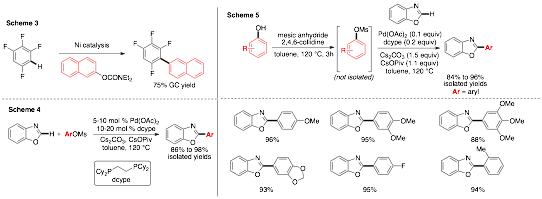
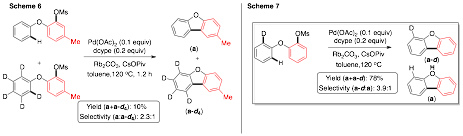
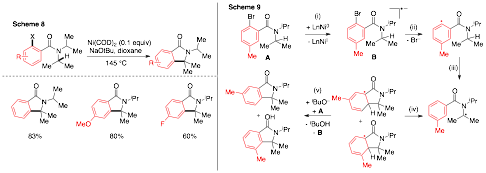
Dipannita Kalyani, PhD, St. Olaf College




Copyright © American Chemical Society