Reports: ND152167-ND1: Chemical and Conformationally Driven Switches in Covalent Gas Separation Materials
Brandon L. Ashfeld, PhD, University of Notre Dame
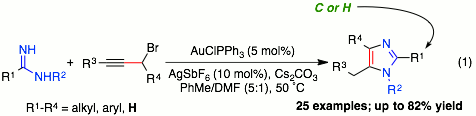 |
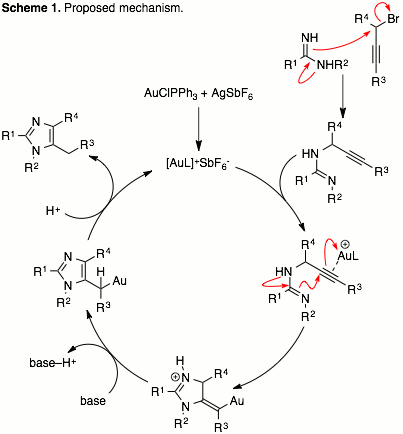
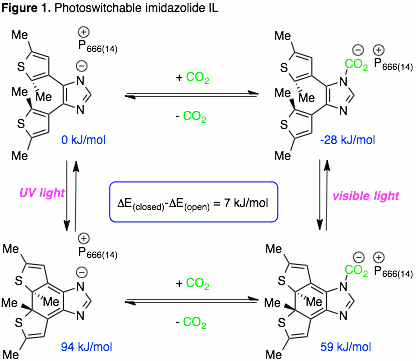
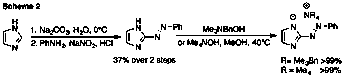
Brandon L. Ashfeld, PhD, University of Notre Dame
 |



Copyright © American Chemical Society