Reports: DNI151705-DNI1: Catalytic Functionalization of Carbon-Hydrogen Bonds with Alkyl Halides
Gong Chen, Ph D, Pennsylvania State University
Key discovery I: Development of Palladium-Catalyzed Picolinamide-Directed Alkylation of Unactivated C(sp3)-H Bonds with Alkyl Iodides
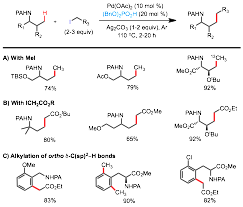
The metal-catalyzed coupling of unactivated sp3 hybridized C-H bonds with alkyl halides remains one of the most difficult challenges in the C-H functionalization field. Advances in this area could offer greatly simplified methods for the construction of C(sp3)-C(sp3) bonds from a large pool of readily accessible and economical starting materials. Despite the progress made on Pd-catalyzed C(sp2)-H alkylation reactions over the past decade, alkylation of unactivated C(sp3)-H bonds with alkyl halides has been much less advanced and protocols with synthetic relevance are even rarer. Under the support of this PRF grant, we have successfully developed a new set of readily applicable Pd-catalyzed reactions to alkylate the unactivated and remote C(sp3)-H bonds of picolinamide-protected aliphatic amines with primary alkyl iodides (Figure 1).ref 1
Key discovery II: Development of Stereoselective Synthesis of β-Alkylated α-Amino Acids via Palladium-Catalyzed Alkylation of Methylene C(sp3)-H Bonds
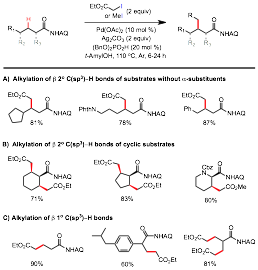
Amino acids are one of nature's most powerful and versatile building blocks for the synthesis of natural products and biomolecules. In addition to the common proteinogenic amino acids, nature uses post-translational modifications to synthesize a myriad of nonproteinogenic amino acids with diverse structures and functions. Among these modifications, alkylation at the β position of α amino acid residues, e.g. C-methyltransferase-mediated methylation, is particularly effective at modulating the conformational and biophysical properties of the parent peptide backbones. These β-alkylated amino acid units contain adjacent carbon stereogenic centers and pose significant synthetic challenge. Complementary to conventional synthesis strategies, we envisioned these molecules could be expeditiously accessed via the selective alkylation of sp3-hybridized C-H bonds on the side chains of simple amino acid precursors. Under the support of this PRF grant, we have discovered a new set of reactions based on the Pd-catalyzed alkylation of unactivated methylene C(sp3)-H bonds of aminoquinolyl aliphatic carboxamides with α-haloacetate and methyl iodide (Figure 2).ref 2 These reactions are highly efficient, versatile, and have broad substrate scope. These reactions represent the first generally applicable method for the catalytic alkylation of unconstrained and unactivated methylene C-H bonds with high synthetic relevance. These reactions enable a streamlined strategy for the synthesis of various natural and unnatural amino acids, particularly β-alkylated α-amino acids, starting from readily available precursors in a diastereoselective manner following a straightforward template.
Key discovery III: Development of a removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated C(sp3)-H bonds
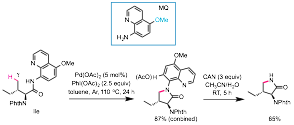
We have developed a new set of reactions for the synthesis of pyrrolidones and indolinones by the palladium-catalyzed carboxamide-directed intramolecular amination of unactivated C(sp3)-H bonds at the γ position of secondary-amide precursors. These reactions harness the unique redox reactivity of six-membered palladacycle intermediates, which are kinetically less favorable than five-membered palladacycles but react readily with PhI(OAc)2 to give γ-lactam products with high selectivity (Figure 3).ref 5 These lactamization reactions are efficient, versatile, and require inexpensive reagents. They can be applied along with other palladium-catalyzed C(sp3)-H functionalization reactions in a sequential manner to quickly transform readily accessible carboxylic acid starting materials into diverse pyrrolidinone products with complex substitution patterns. We have also introduced the MQ auxiliary, which can be removed readily and greatly improves the synthetic utility of these reactions by enabling applications in the synthesis of complex molecules.
List of publication which acknowledged the support of ACS-PRF:
1. Shuyu Zhang, Gang He, William A. Nack, Yingsheng Zhao, Qiong Li, Gong Chen* Palladium-Catalyzed Picolinamide-Directed Alkylation of Unactivated C(sp3)-H Bonds with Alkyl Iodides. J. Am. Chem. Soc. 2013, 135, 2124-2127.
2. Shu-Yu Zhang, Qiong Li, Gang He, William A. Nack, Gong Chen* Stereoselective Synthesis of β-Alkylated α-Amino Acids via Palladium-Catalyzed Alkylation of Methylene C(sp3)-H Bonds. J. Am. Chem. Soc. 2013, 135, 12135-12141.
3. Ryan Pearson, Shuyu Zhang, Gang He, Nicola Edwards, and Gong Chen*. Synthesis of Phenanthridines via Palladium-Catalyzed Picolinamide-Directed Sequential C-H Functionalization. Beilstein J. Org. Chem. 2013, 9, 891-899.
4. William A. Nack, Gang He, Shu-Yu Zhang, Chengxi Lu, and Gong Chen*. Iodination of Remote ortho-C-H Bonds of Arenes via Picolinamide-Directed Electrophilic Aromatic Substitution: A Streamlined Synthesis Strategy for Tetrahydroquinolines. Org. Lett. 2013, 15, 3440-3443.
5. Gang He, Shu-Yu Zhang, William A. Nack, and Gong Chen*. Use of a Readily Removable Auxiliary Group for the Synthesis of Pyrrolidones by the Palladium-Catalyzed Intramolecular Amination of Unactivated γ C(sp3)-H Bonds. Angew. Chem. Int. Ed. 2013, 52, 11124-11128.
6. Qiong Li, Shu-Yu Zhang, Gang He, Zhaoyan Ai, William A. Nack, and Gong Chen*. Copper-Catalyzed Picolinamide-Directed ortho Amination of ortho-C-H Bonds of Anilines with Alkylamines at Room Temperature. Org. Lett. 2014, 16, 1764-1767
7. Bo Wang, William A. Nack, Gang He, Shuyu Zhang, and Gong Chen*. Palladium-Catalyzed Trifluoroacetate-Promoted Mono-Arylation of ß Methyl Group of Alanine at Room Temperature: Synthesis of ß-Arylated a-Amino Acids through Sequential C-H Functionalization. Chem. Sci. 2014, 5, 3952-3957.
Figure 1. Pd-catalyzed PA-directed Alkylation of γ C(sp3)-H bonds
Figure 2. Pd-catalyzed AQ-directed Alkylation of β C(sp3)-H bonds
Figure 3. Pd-catalyzed Intramolecular Amination of γ C(sp3)-H bonds