Reports: DNI751049-DNI7: Curvature-Directed Crystallization of Polymer Dielectrics
Jodie Lutkenhaus, Texas A&M University
The scientific objective of this project is to enhance and control the dielectric properties of popularly used polymer dielectrics (isotactic poly(propylene), poly(vinylidene fluroide), poly(ethylene terepthalate)) via curvature-directed crystallization, which is the crystallization of a polymer confined within a cylindrical nanoscale pore. Our rationale is that curvature-directed crystallization may produce oriented, homogeneous crystallites of improved dielectric properties (lower loss, higher breakdown strength) relative to bulk. Although confinement effects within polymer thin planar films are well-studied, less is known about the behavior of polymer dielectrics in confined cylindrical geometries.
We have completed an investigation on the curvature-directed crystallization of isotactic poly(propylene), and we initiated a similar investigation on poly(carbonate). Our results on poly(propylene) have been published in Journal of Polymer Science Part B: Polymer Physics, and we are currently preparing a manuscript on polycarbonate.
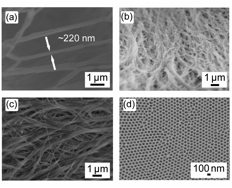
First, the formation of isotactic poly(propylene) (iPP) nanowires of tunable diameter was demonstrated by melt-wetting iPPinto nanoporous anodic alumina. Figure 1 shows nanowires of varying diameters and an example of an empty alumina template.
Figure 1. Scanning electron microscope (SEM) images of nanowires formed via melt-wetting of iPPinto nanoporous AAO after template removal; structures derived from pore diameters of (a) 200 nm, (b) 40 nm, and (c) 15 nm are shown. (d) Top-view of empty 40 nm pore diameter AAO templates.
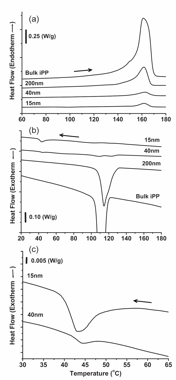
Then, the crystallization process was analyzed using differential scanning calorimetry for iPP-loaded alumina, Figure 2. A transition from hetero- to homogeneous crystallization was observed as the pore diameter decreased. This is distinguished by a shift to lower temperatures and a broadening of the crystallization peak (Figure 2b). Non-isothermal crystallization was also investigated, and Avrami analysis was applied. The Avrami exponent decreased as the pore diameter decreased, indicating preferential 1-D crystallization for diameters of 40 nm or less.This is a significant finding because it demonstrates that crystallization occurs quite differently over varying time scales due to the "freezing out" of crystals growing as the impinge against the nanopore wall.
Figure 2. Heating (a) and cooling (b) DSC scans of bulk-iPP, iPP-200, iPP-40 and iPP-15; (c) an expanded view of the low-temperature exothermic peaks. The scan rate was 10 oC/min, and the second scan is shown.
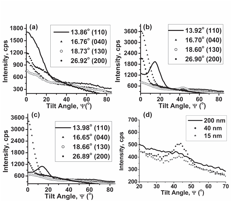
X-ray diffraction (XRD) studies, Figure 3, showed that the iPPcrystallizes into the a-phase and that it preferentially orients along the a- and b-axis, perpendicular to the pore wall. By using XRD with texture analysis (with a cradle), distinct peaks were observed in Ψ-XRD patterns. This result further confirms the presence of oriented crystals arising from one-dimensional crystallization.
Figure 3. Ψ-XRD patterns of (a) iPP-200, (b) iPP-40 and (c) iPP-15 for various 2ϴ values. (d) An expanded view of the patterns when 2ϴ is 18.65 o, corresponding to (130) planes.
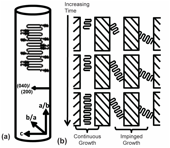
From these results we can now provide a full picture for how confined geometries, specifically cylindrical geometries, control the crystallization of iPP. Figure 4 a is a schematic representation of the proposed orientation of the polymer chains inside the 40 nm and 15 nm diameter nanopores. As the polymer is placed in increasingly stringent cylindrical confinement the chains preferentially crystallize in the direction of the a- and b-axes. In other words, the polymer chains are oriented perpendicular to the pore's axis. Similarly, the (040) and (200) family of planes are normal to the nanopore long axis and parallel to the template surface. Figure 4 b illustrates the progression of crystallite growth with time. At the beginning of crystal growth polymer chains are randomly oriented throughout the nanopore (top row). With time, as growth progresses, only chains oriented parallel to the nanopore wall continue to grow (middle row). All other chains are physically impinged by the nanopore walls(middle and bottom row). For polymer confined in the template bearing 200 nm diameter pores, this effect does not occur due to the reduced surface area and decreased radius of curvature. These results show that curvature-directed crystallization is a powerful means to control a polymer's crystallization and orientation.
Figure 4. (a) Schematic illustration of the iPP chain orientation relative to the long axis of the nanopore wall. (b) Schematic view of the orientation of the iPPcrystallites formed inside AAO templates in relation to the pore wall.
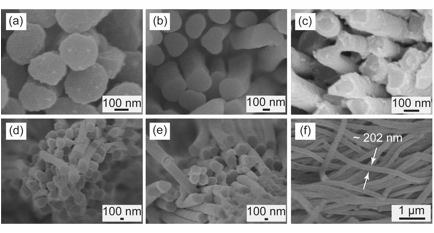
Most recently, we have demonstrated the infiltration of polycarbonate into nanoporous AAO. We found the melt-wetting process to be quite challenging for this polymer, so we initiated an investigation regarding the melt-wetting process as a function of AAO surface chemistry. AAO templates were modified with alkane- and fluorosilanes, and the melt-wetting and thermal behavior of the resulting polycarbonate nanowires was determined. Figure 5 shows polycarbonate nanotubes obtained from alkane- and fluorosilane-modified AAO templates. Note that the polycarbonate-AAO contact angle, as evidenced by the tips of the nanowires, varies depending on the silanetype. Thermal analysis of these tubes revealed that the glass transition temperature shifts only slightly depending on the surface chemistry. Crystallization was largely suppressed for this system.
Figure 5. Polycarbonate nanotubes released from (a-b) floursilane-treated AAO, (c) native AAO, and (d-e) alkanesilane-treated AAO. (f) Polycarbonate nanowires show diameter comparable to their original template.