Reports: ND150808-ND1: New Studies in Alkene 'Pseudohalogen' Difunctionalization
Thomas Lectka, PhD, Johns Hopkins University
Scheme 1.
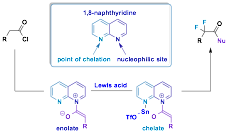
In very fruitful work on difluorination, a dually activated ketene enolate, generated from an acid chloride, the unusual chelating nucleophile (1,8-naphthyridine) and a Lewis acid, reacts to afford a host of α,α-difluorinated products in the presence of a bench top stable fluorinating agent (Selectfluor). The use of this method to synthesize otherwise difficult to make products is highlighted in a publication along with computational and spectroscopic support for the proposed chelate (Scheme 2).
Scheme 2.
These results mark the end of funding for this grant. Although we deviated from the original proposed research, the end results were more important than the originals would have ever been. We are therefore grateful to the ACS-PRF for its support.