Reports: UR650670-UR6: Petroleomics: Elucidation of Chemical Composition and Functionality of Compounds Resistant to Asphaltene Inhibitors
Geoffrey C. Klein, PhD, Christopher Newport University
This ACS-PRF grants supports laboratory work on the characterization of crude oils, specifically the maltene fraction, for the determination of those compounds that might enhance the stability of the asphaltene fraction. The use of several asphaltene flocculation inhibitors has been used to test for the enhancement of asphaltene stability. The specific aims of this study are to fully characterize the maltene fraction (the saturates, aromatic, and resin fractions) and determine the effect of asphaltene flocculation inhibitors on the stability of asphaltenes. Low-resolution mass spectrometry, Fourier Transform Ion Cyclotron Resonance Spectrometry, and several spectroscopic techniques have been employed for the determination of a sample's elemental and functional group composition, Hildebrand solubility, and the effectiveness of several different asphaltene inhibitors.
Research Progress
Work this past year has focused on the determination of the Hildebrand solubility for the crude oils and their different fractions. Table 1 illustrates the Hildebrand solubility parameters (HSP) for the different oils—light, medium, and heavy—and their fractions. This solubility parameter can be used as a means to predict the probability of asphaltene flocculation. Hildebrand indicated that the maximum stability is observed when the solute and solvent cohesive energy densities are similar. Our data suggest that the asphaltene and maltene fractions indicate an increased probability for asphaltene flocculation for all three oils. In addition, the HSP of the aromatic fractions most closely matched that of the asphaltene fraction—a fraction that might be used as a flocculation inhibitor to reduce the probability of precipitation. These HSPs correlate well with other bulk properties reported in previous reports (e.g. H:C ratio).High-resolution mass spectrometry is currently being used to characterize the fractions in an effort to link elemental composition to the Hildebrand solubility parameters and other bulk properties.
| Table 1. Hildebrand Solubility Parameters the Crude Oils and the SARA Fractions. | |||||
| Oil | Maltene* (MPa1/2) | Asphaltene (MPa1/2) | Saturates (MPa1/2) | Aromatics (MPa1/2) | Resins (MPa1/2) |
| Light, A | 17.8 | 20.9 | 17.3 | 20.5 | 19.6 |
| Medium, C | 17.8 | 19.9 | 17.4 | 19.8 | 16.0 |
| Heavy, B | 18.4 | 20.6 | 18.1 | 20.0 | 17.2 |
| *Defined as the Saturate, Aromatic and Resin Fractions | |||||
Aim 1
Characterization of the elemental and function group composition of the whole crude oils has largely been reported in previous reports. A grant was recently submitted to the National High Magnetic Field Laboratory (NHMFL) for the characterization of the maltene fractions to draw conclusions on the composition of this fraction and its ability to stabilize the asphaltene fraction.
Aim 2
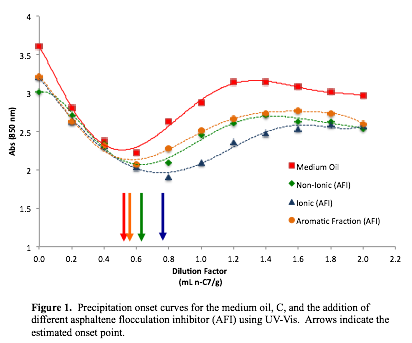
Most of our work has focused on the use of different asphaltene flocculation inhibitors (AFI) to enhance the stability of the asphaltene fraction. Figure 1 illustrates the effectiveness of the different types of inhibitors—ionic, non-ionic, and aromatic fraction—on the medium crude oil (C). The flocculation onset point, indicated by the minimum of the curve, is representative of the stability of the asphaltene fraction within the maltene fraction. The ionic inhibitor has the greatest impact on the stability of the asphaltene fraction, as shown by the increase in the flocculation onset point (indicated by the blue arrow). Once possible explanation for the increased stability of the asphaltene fraction in the presence of the ionic inhibitor is the formation of micelles between the ionic inhibitor and the asphaltene molecules. The other inhibitors only had a minor effect on the stability of the asphaltene fraction.
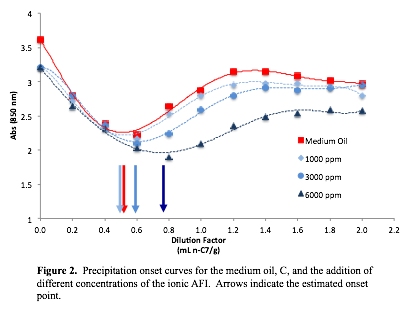
Additional experiments varying the concentrations of the ionic inhibitor were performed. Figure 2 illustrates the precipitation onset curves when the concentration of the ionic inhibitor is increased. As predicted, the higher ionic inhibitor concentration enhanced the stability of the asphaltene fraction. The moderate amount of asphaltenes in Oil C (~2%) might explain why the lower concentration of AFI had no effect on the stability of the asphaltenes. Additional precipitation curves will be produced in an attempt to correlate the percent asphaltene content and the necessary concentration of AFI to stabilize the asphaltene fraction.
Conclusion
Work towards the completion of the specific aims of this grant continue and should be nearing completion at the end of the next year. Several techniques still need to be employed to complete the analysis of the multiple maltene fractions and precipitation onset curves. Overall, this grant has funded seven undergraduate students, and exposed several other students to research in the petroleum field. The Klein research group has presented several times at regional and national conference—including six presentations at the ACS and Pittcon national meetings. We are nearing completion of two manuscripts as a direct result of the work funded by ACS-PRF. Future work will build upon the correlation of the HSP and crude oil content.