Reports: UR151036-UR1: Applications of Iron(III) Compounds as Catalysts in Organic Synthesis
Ram S. Mohan, Illinois Wesleyan University
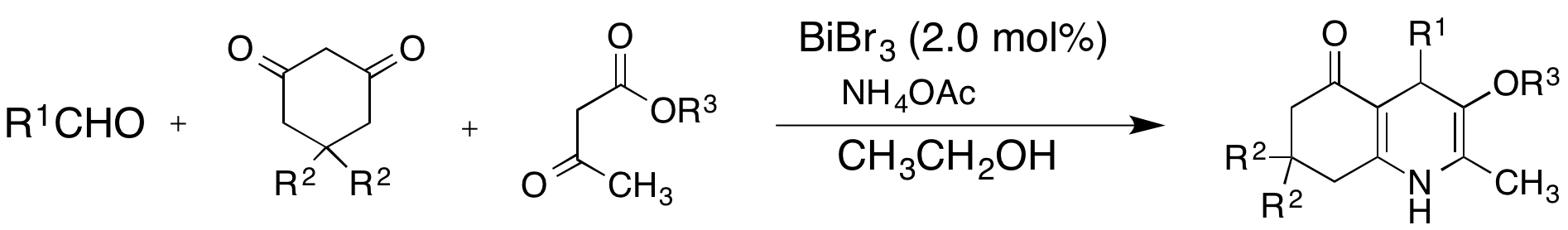
We first investigated the iron(III) tosylate catalyzed one-pot synthesis of polyhydroquinolines via a multicomponent synthesis (The Hantzsch reaction). However, initial results were not as promising as they were for some other reactions carried out in the group using iron(III) tosylate. Due to our interest in polyhydroquinoline, we continued screening other catalysts and found that the best results were obtained with bismuth(III) bromide. All reactions were carried out at room temperature or 50° C when slow at room temperature. Product was obtained in good to excellent yields after recrystallization or simply washing with ethanol/water (1:1). The use of a non toxic bismuth salt, and a relatively green solvent, ethanol make this method particularly attractive from a green chemistry perspective. These results are quite promising and we expect to publish a manuscript based on this work in the near future.
Table 1. Synthesis of polyhydroquinoline derivatives catalyzed by BiBr3
Entry |
R1 |
R2 |
R3 |
Time and T (°C) |
Yield (%) |
||||||
1 |
p-CH3 |
CH3 |
CH3CH2 |
3.5 h, rt |
87 |
||||||
2 |
p-OCH3 |
CH3 |
CH3CH2 |
23 h, rt |
87 |
||||||
3 |
p-N(CH3)2 |
CH3 |
CH3CH2 |
4 h, 50° C |
86 |
||||||
4 |
p-Cl |
CH3 |
CH3CH2 |
2.5, rt |
93 |
||||||
5 |
p-NO2 |
CH3 |
CH3CH2 |
17 h, rt |
87 |
||||||
6 |
m-OCH3 |
CH3 |
CH3CH2 |
2 h, 50° C |
90 |
||||||
7 |
m-CH3 |
CH3 |
CH3CH2 |
5.5, rt |
86 |
||||||
8 |
p-NO2 |
CH3 |
CH3CH2 |
17 h, rt |
78 |
||||||
9 |
2-Furyl |
CH3 |
CH3CH2 |
4 h. rt |
87 |
||||||
10 |
p-C6H4 |
CH3 |
CH3CH2 |
3.5, 50° C |
91 |