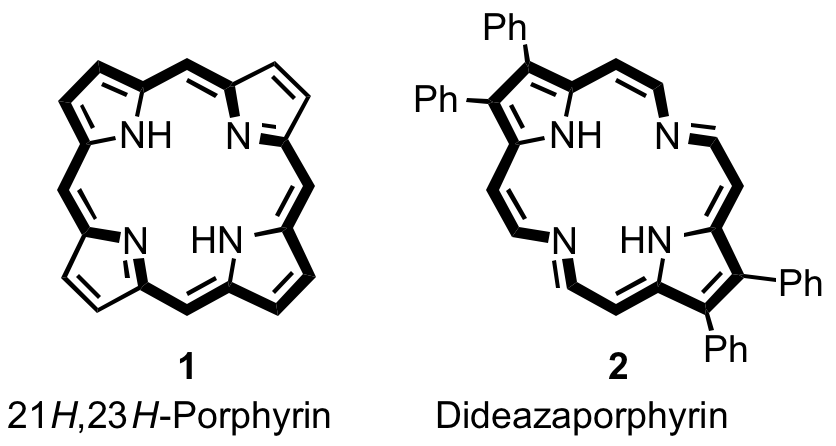
Reports: UR152798-UR1: Synthesis of Azaannulenes Related to the Porphyrins
Timothy D. Lash, Illinois State University
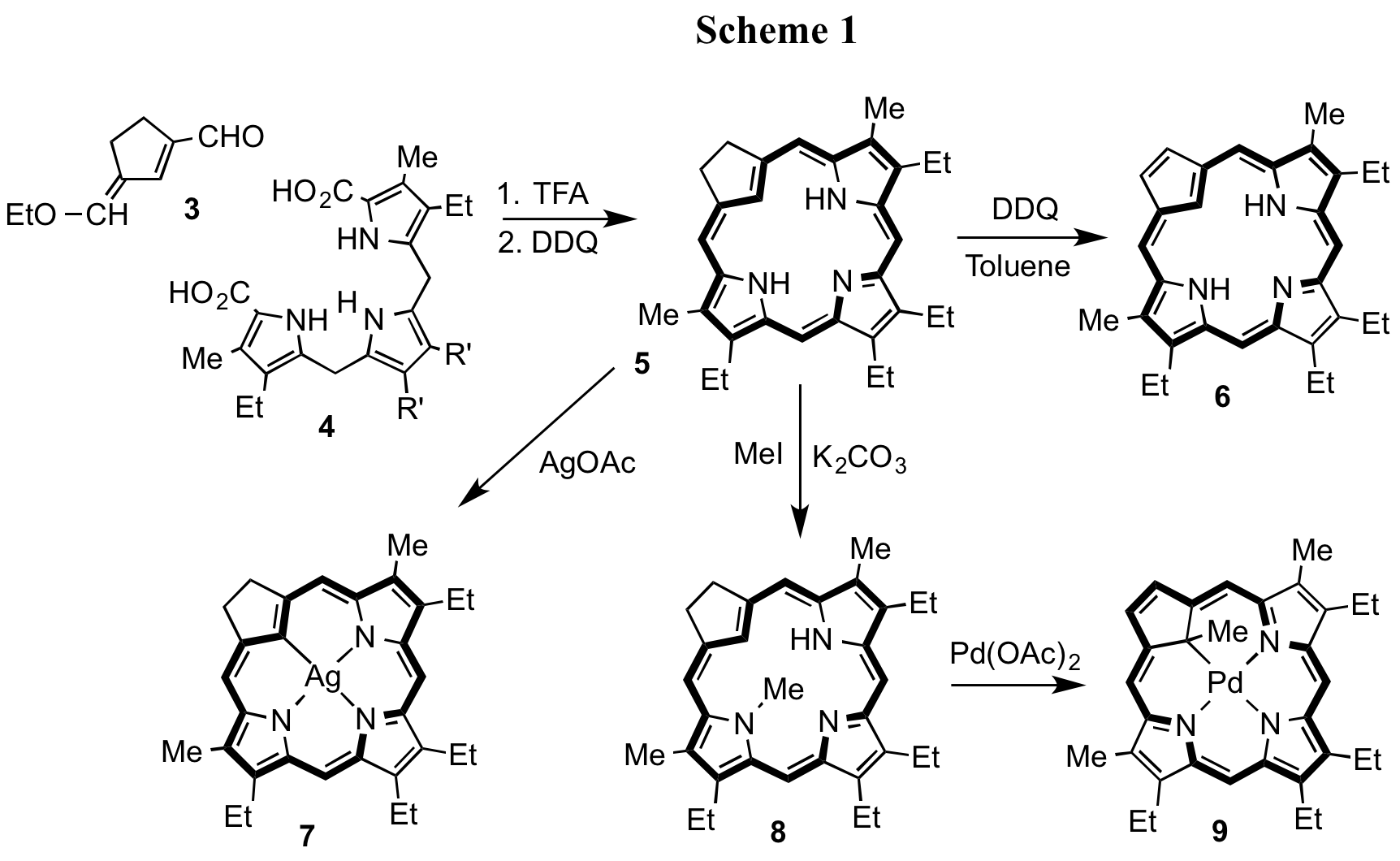
Carbaporphyrins with unsubstitued cyclopentadienyl rings had not been prepared previously, and a synthetic route to this system has been developed.7 Condensation of 3 with tripyrrane 4 in the presence of TFA, followed by oxidation with aqueous ferric chloride, gave carbachlorin 5 in 11-16% yield (Scheme 1). Subsequent oxidation with DDQ afforded carbaporphyrin 6.7 Carbaporphyrin 6 exhibited a strong diamagnetic ring current by proton NMR spectroscopy and the internal CH resonance was shifted upfield to -6.9 ppm.7 However, the UV-vis spectrum for 6 differed significantly from true porphyrins and gave broad absorptions in the Soret band region. Interestingly, carbachlorin 5 gave a much more porphyrin-like UV-vis spectrum, exhibiting a strong Soret band at 401 nm.7 In addition, the proton NMR spectrum for 5 showed that this species is highly diatropic and the internal CH resonance was noted at -6.8 ppm. Carbachlorin 5 reacted with silver(I) acetate to give the corresponding silver(III) complex 7, while treatment with methyl iodide and potassium carbonate in refluxing acetone gave the N-methyl derivative 8. Subsequent reaction with palladium(II) acetate in acetonitrile resulted in a sequential metalation-oxidation-alkyl group migration sequence to give palladium(II) carbaporphyrin 9.7 This organometallic derivative retained strongly aromatic properties, and the proton NMR spectrum showed the resonance for the interior methyl unit upfield at -4.46 ppm.7
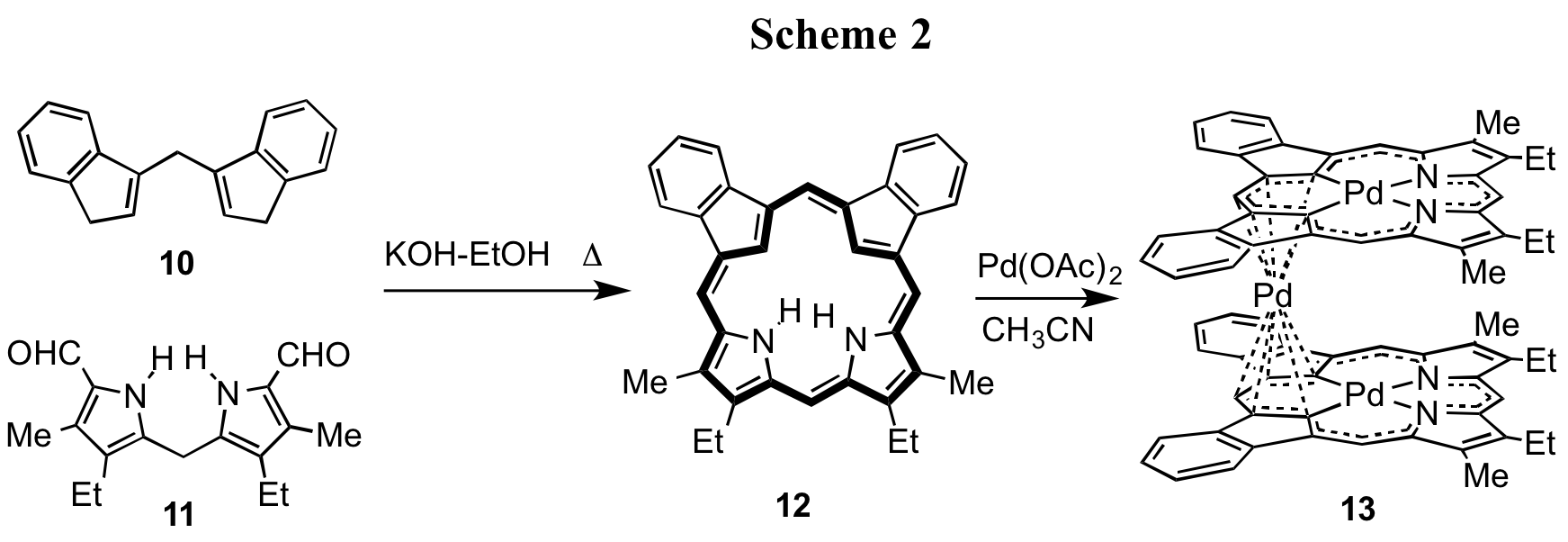
Dicarbaporphyrinoid systems are far less well studied than the monocarbaporphyrinoids, although several examples have been described.8,9 In order to pursue further investigations into these promising porphyrinoid systems, we have turned to the application of diindenylmethane 109 as an intermediate for dicarbaporphyrin synthesis (Scheme 2). Reaction of 10 with dipyrrylmethane dialdehyde 11 in refluxing 1% KOH-ethanol generated adj-dicarbaporphyrin 12 in 21% yield.11 Porphyrin analogue 12 was also shown to retain highly diatropic characteristics, and the proton NMR spectrum in CDCl3 showed the internal CH protons at -6.24 ppm, while the meso-protons gave rise to three singlets at 8.91 (1H), 9.09 (2H) and 9.48 ppm (1H).11 Metalation of 12 with Pd(OAc)2 gave an unprecedented organometallic sandwich complex 13 where two palladium(II) dicarbaporphyrin anions enclosed a Pd(IV) anion.11 Therefore, these studies are demonstrating that modified structures of this type exhibit many porphyrin-like properties, but also have many unique properties of their own.
References
1. Lash, T. D. Recent advances in the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems, Eur. J. Org. Chem. 2007, 5461-5481.
2. Lash, T. D.; Jones, S. A.; Ferrence, G. M. Synthesis and characterization of tetraphenyl-21,23-dideazaporphyrin: the best evidence yet that porphyrins really are the [18]annulenes of nature, J. Am. Chem. Soc. 2010, 132 (37), 12786-12787.
3. Lash, T. D. Carbaporphyrinoids: Taking the Heterocycle Out of Nature's [18]Annulene, Synlett 2000, 279-295.
4. Lash, T. D. Origin of Aromatic Character in Porphyrinoid Systems, Journal of Porphyrins and Phthalocyanines 2011, 15, 1093-1115.
5. AbuSalim, D. I.; Lash, T. D. Relative stability and diatropic character of carbaporphyrin, dicarbaporphyrin, tricarbaporphyrin and quatyrin tautomers, J. Org. Chem. 2013, 78, 11535-11548.
6. AbuSalim, D. I.; Lash, T. D. Aromatic character and stability of neo-confused porphyrin tautomers and related compounds, Org. Biomol. Chem. 2013, 11, 8306-8323.
7. Li, D.; Lash, T. D. Synthesis and reactivity of carbachlorins and carbaporphyrins, J. Org. Chem. 2014, 79, 7112-7121.
8. a. Lash, T. D.; Romanic, J. L.; Hayes, M. J.; Spence, J. D. Towards Hydrocarbon Analogues of the Porphyrins: Synthesis and Spectroscopic Characterization of the First Dicarbaporphyrin, Chem. Commun. 1999, 819-820. b. Graham, S. R.; Colby, D. A.; Lash, T. D. Azulene analogues of tripyrranes and carbaporphyrinoids therefrom, Angew. Chem. Int. Ed. 2002, 41, 1371-1374. c. Xu, L.; Lash, T. D., Synthesis of aromatic dicarbaporphyrinoids from resorcinol and 2-methyl-resorcinol, Tetrahedron Lett. 2006, 47, 8863-8866.
9. a. Lash, T. D.; Colby, D. A.; Idate, A. S.; Davis, R. N., Fulvene dialdehyde strategy for adj-dicarba-porphyrinoid synthesis: preparation of a 22-carbaazuliporphyrin, J. Am. Chem. Soc. 2007, 129, 13801-13802. b. Lash, T. D.; Lammer, A. D.; Idate, A. S.; Colby, D. A.; White, K. Preparation of azulene-derived fulvenedialdehydes and their application to the synthesis of stable adj-dicarbaporphyrinoids, J. Org. Chem. 2012, 77, 2368-2381. c. Lash, T. D.; Lammer, A. D.; Ferrence, G. M. Two-step synthesis of stable dioxadicarbaporphyrins from bis(3-indenyl)methane, Angew. Chem. Int. Ed. 2012, 51, 10871-10875. d. AbuSalim, D. I.; Merfeld, M. L.; Lash, T. D. Dicarbaporphyrinoid systems. Synthesis of oxo-adj-dibenziphlorins, J. Org. Chem. 2013, 78, 10360-10368.
10. Li, H.; Stern, C. L.; Marks, T. J. Significant proximity and cocatalyst effects in binuclear catalysis for olefin polymerization, Macromolecules 2005, 38, 9015-9027.
11. AbuSalim, D. I.; Ferrence, G. M.; Lash, T. D. Synthesis of an adj-dicarbaporphyrin and the formation of an unprecedented tripalladium sandwich complex, J. Am. Chem. Soc. 2014, 136, 6763-6772.