Reports: UNI152318-UNI1: The Synthesis of C4 Symmetric Oxacalix[4]arenes and Related Macrocycles
Jay Wm. Wackerly, PhD, Central College
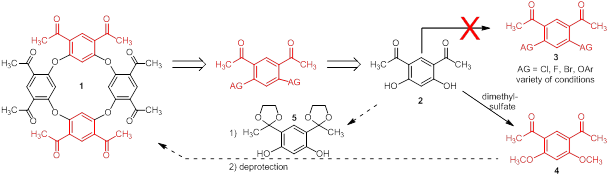
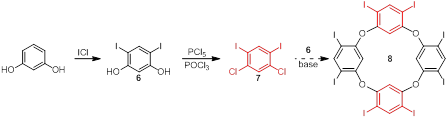
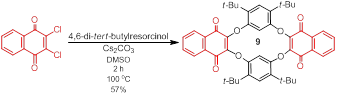
Jay Wm. Wackerly, PhD, Central College



Copyright © American Chemical Society